The glucocorticoid receptor: cause of or cure for obesity?
- PMID: 26714851
- PMCID: PMC4838130
- DOI: 10.1152/ajpendo.00478.2015
The glucocorticoid receptor: cause of or cure for obesity?
Abstract
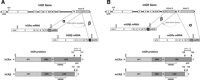
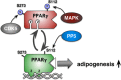
Glucocorticoid hormones (GCs) are important regulators of lipid metabolism, promoting lipolysis with acute treatment but lipogenesis with chronic exposure. Conventional wisdom posits that these disparate outcomes are mediated by the classical glucocorticoid receptor GRα. There is insufficient knowledge of the GC receptors (GRα and GRβ) in metabolic conditions such as obesity and diabetes. We present acute models of GC exposure that induce lipolysis, such as exercise, as well as chronic-excess models that cause obesity and lipid accumulation in the liver, such as hepatic steatosis. Alternative mechanisms are then proposed for the lipogenic actions of GCs, including induction of GC resistance by the GRβ isoform, and promotion of lipogenesis by GC activation of the mineralocorticoid receptor (MR). Finally, the potential involvement of chaperone proteins in the regulation of adipogenesis is considered. This reevaluation may prove useful to future studies on the steroidal basis of adipogenesis and obesity.
Keywords: adipogenesis; adipose differentiation; glucocorticoid receptor; glucocorticoid receptor-α; glucocorticoid receptor-β; glucocorticoids; lipolysis.
Copyright © 2016 the American Physiological Society.
Figures


References
-
- Adams M, Reginato MJ, Shao D, Lazar MA, Chatterjee VK. Transcriptional activation by peroxisome proliferator-activated receptor gamma is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. J Biol Chem 272: 5128–5132, 1997. - PubMed
-
- Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, Evans RM. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science 237: 268–275, 1987. - PubMed
-
- Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell 4: 585–595, 1999. - PubMed
-
- Berson A, De Beco V, Letteron P, Robin MA, Moreau C, El Kahwaji J, Verthier N, Feldmann G, Fromenty B, Pessayre D. Steatohepatitis-inducing drugs cause mitochondrial dysfunction and lipid peroxidation in rat hepatocytes. Gastroenterology 114: 764–774, 1998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

