Impact of Tumor Size on Probability of Pathologic Complete Response After Neoadjuvant Chemotherapy
- PMID: 26714960
- PMCID: PMC4819747
- DOI: 10.1245/s10434-015-5030-1
Impact of Tumor Size on Probability of Pathologic Complete Response After Neoadjuvant Chemotherapy
Abstract
Background: The prospective Neoadjuvant Breast Symphony Trial (NBRST) study found that MammaPrint/BluePrint functional molecular subtype is superior to conventional immunohistochemistry/fluorescence in situ hybridization subtyping for predicting pathologic complete response (pCR) to neoadjuvant chemotherapy. The purpose of this substudy was to determine if the rate of pCR is affected by tumor size.
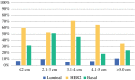
Methods: The NBRST study includes breast cancer patients who received neoadjuvant chemotherapy. MammaPrint/BluePrint subtyping classified patients into four molecular subgroups: Luminal A, Luminal B, HER2 (human epidermal growth factor receptor 2), and Basal type. Probability of pCR (ypT0/isN0) as a function of tumor size and molecular subgroup was evaluated.
Results: A total of 608 patients were evaluable with overall pCR rates of 28.5 %. Luminal A and B patients had significantly lower rates of pCR (6.1 and 8.7 %, respectively) than either basal (37.1 %) or HER2 (55.0 %) patients (p < 0.001). The probability of pCR significantly decreased with tumor size >5 cm [p = 0.022, odds ratio (OR) 0.58, 95 % confidence interval (CI) 0.36, 0.93]. This relationship was statistically significant in the Basal (p = 0.026, OR 0.46, 95 % CI 0.23, 0.91) and HER2 (p = 0.039, OR 0.36, 95 % CI 0.14, 0.95) subgroups. In multivariate logistic regression analyses, the dichotomized tumor size variable was not significant in any of the molecular subgroups.
Discussion: Even though tumor size would intuitively be a clinical determinant of pCR, the current analysis showed that the adjusted OR for tumor size was not statistically significant in any of the molecular subgroups. Factors significantly associated with pCR were PR status, grade, lymph node status, and BluePrint molecular subtyping, which had the strongest correlation.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous