Kinetics of the inhibition of renin and angiotensin I-converting enzyme by cod (Gadus morhua) protein hydrolysates and their antihypertensive effects in spontaneously hypertensive rats
- PMID: 26715103
- PMCID: PMC4695624
- DOI: 10.3402/fnr.v59.29788
Kinetics of the inhibition of renin and angiotensin I-converting enzyme by cod (Gadus morhua) protein hydrolysates and their antihypertensive effects in spontaneously hypertensive rats
Abstract
Background: Cod muscle has a balanced protein profile that contains potentially bioactive amino acid sequences. However, there is limited information on release of these peptides from the parent proteins and their ability to modulate mammalian blood pressure.
Objective: The aim of this study was to generate cod antihypertensive peptides with potent in vitro inhibitory effects against angiotensin-converting enzyme (ACE) and renin. The most active peptides were then tested for systolic blood pressure (SBP)-reducing ability in spontaneously hypertensive rats (SHRs).
Design: Cod protein hydrolysate (CPH) was produced by subjecting the muscle proteins to proteolysis first by pepsin and followed by trypsin+chymotrypsin combination. In order to enhance peptide activity, the CPH was subjected to reverse-phase (RP)-HPLC separation to yield four fractions (CF1, CF2, CF3, and CF4). The CPH and RP-HPLC fractions were each tested at 1 mg/mL for ability to inhibit in vitro ACE and renin activities. CPH and the most active RP-HPLC fraction (CF3) were then used for enzyme inhibition kinetics assays followed by oral administration (200 and 30 mg/kg body weight for CPH and CF3, respectively) to SHRs and SBP measurements within 24 h.
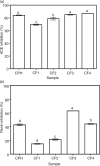
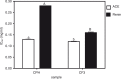
Results: The CPH, CF3, and CF4 had similar ACE-inhibitory activities of 84, 85, and 87%, which were significantly (p<0.05) higher than the values for CF1 (69%) and CF2 (79%). Conversely, the CF3 had the highest (63%) renin-inhibitory activity (p<0.05) when compared to CPH (43%), CF1 (15%), and CF4 (44%). CPH and CF3 exhibited uncompetitive mode of ACE inhibition, whereas renin inhibition was non-competitive. Even at a 6.7-fold lower dosage, the CF3 significantly (p<0.05) reduced SBP (maximum -40.0 mmHg) better than CPH (maximum -19.1 mmHg).
Conclusions: RP-HPLC fractionation led to enhanced antihypertensive effects of cod peptides, which may be due to a stronger renin-inhibitory activity.
Keywords: IC50; angiotensin I-converting enzyme; cod; enzyme inhibition kinetics; protein hydrolysate; renin; spontaneously hypertensive rats; systolic blood pressure.
Figures



Similar articles
-
Evaluation of the in vitro antioxidant properties of a cod (Gadus morhua) protein hydrolysate and peptide fractions.Food Chem. 2015 Apr 15;173:652-9. doi: 10.1016/j.foodchem.2014.10.079. Epub 2014 Oct 22. Food Chem. 2015. PMID: 25466072
-
Antihypertensive properties of tilapia (Oreochromis spp.) frame and skin enzymatic protein hydrolysates.Food Nutr Res. 2017 Oct 24;61(1):1391666. doi: 10.1080/16546628.2017.1391666. eCollection 2017. Food Nutr Res. 2017. PMID: 29151830 Free PMC article.
-
Isolation of novel ACE-inhibitory peptide from naked oat globulin hydrolysates in silico approach: Molecular docking, in vivo antihypertension and effects on renin and intracellular endothelin-1.J Food Sci. 2020 Apr;85(4):1328-1337. doi: 10.1111/1750-3841.15115. Epub 2020 Mar 27. J Food Sci. 2020. PMID: 32220144
-
Antihypertensive peptides from food proteins.Annu Rev Food Sci Technol. 2015;6:235-62. doi: 10.1146/annurev-food-022814-015520. Annu Rev Food Sci Technol. 2015. PMID: 25884281 Review.
-
Amaranth as a Source of Antihypertensive Peptides.Front Plant Sci. 2020 Sep 25;11:578631. doi: 10.3389/fpls.2020.578631. eCollection 2020. Front Plant Sci. 2020. PMID: 33101347 Free PMC article. Review.
Cited by
-
Effects of intact and hydrolysed blue whiting proteins on blood pressure and markers of kidney function in obese Zucker fa/fa rats.Eur J Nutr. 2021 Feb;60(1):529-544. doi: 10.1007/s00394-020-02262-9. Epub 2020 May 14. Eur J Nutr. 2021. PMID: 32409916 Free PMC article.
-
A novel angiotensin I-converting enzyme inhibitory peptide derived from the trypsin hydrolysates of salmon bone proteins.PLoS One. 2021 Sep 2;16(9):e0256595. doi: 10.1371/journal.pone.0256595. eCollection 2021. PLoS One. 2021. PMID: 34473745 Free PMC article.
-
Bioactive Properties of Enzymatic Gelatin Hydrolysates Based on In Silico, In Vitro, and In Vivo Studies.Molecules. 2024 Sep 16;29(18):4402. doi: 10.3390/molecules29184402. Molecules. 2024. PMID: 39339395 Free PMC article.
-
Anti-hypertensive effect of enriched white melon seed protein concentrate biscuit on sodium fluoride exposed rats.World J Exp Med. 2025 Jun 20;15(2):105798. doi: 10.5493/wjem.v15.i2.105798. eCollection 2025 Jun 20. World J Exp Med. 2025. PMID: 40546665 Free PMC article.
-
Production and characterization of chicken blood hydrolysate with antihypertensive properties.Poult Sci. 2020 Oct;99(10):5163-5174. doi: 10.1016/j.psj.2020.07.006. Epub 2020 Jul 27. Poult Sci. 2020. PMID: 32988556 Free PMC article.
References
-
- Jensen IJ, Eysturskarð J, Madetoja M, Eilertsen K-E. The potential of cod hydrolyzate to inhibit blood pressure in spontaneously hypertensive rats. Nutr Res. 2014;34:168–73. - PubMed
-
- Himaya SWA, Ngo DH, Ryu B, Kim SK. An active peptide purified from gastrointestinal enzyme hydrolysate of Pacific cod skin gelatin attenuates angiotensin-1 converting enzyme (ACE) activity and cellular oxidative stress. Food Chem. 2012;132:1872–82.
-
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–23. - PubMed
-
- WHO. World Health Day 2013. Available from: http://www.emro.who.int/world-health-days/2013/nutrition-hypertension-fa... [cited 4 December 2015].
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

