Insulated Foamy Viral Vectors
- PMID: 26715244
- PMCID: PMC4800274
- DOI: 10.1089/hum.2015.110
Insulated Foamy Viral Vectors
Abstract
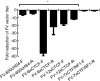
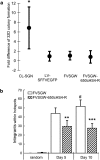
Retroviral vector-mediated gene therapy is promising, but genotoxicity has limited its use in the clinic. Genotoxicity is highly dependent on the retroviral vector used, and foamy viral (FV) vectors appear relatively safe. However, internal promoters may still potentially activate nearby genes. We developed insulated FV vectors, using four previously described insulators: a version of the well-studied chicken hypersensitivity site 4 insulator (650cHS4), two synthetic CCCTC-binding factor (CTCF)-based insulators, and an insulator based on the CCAAT box-binding transcription factor/nuclear factor I (7xCTF/NF1). We directly compared these insulators for enhancer-blocking activity, effect on FV vector titer, and fidelity of transfer to both proviral long terminal repeats. The synthetic CTCF-based insulators had the strongest insulating activity, but reduced titers significantly. The 7xCTF/NF1 insulator did not reduce titers but had weak insulating activity. The 650cHS4-insulated FV vector was identified as the overall most promising vector. Uninsulated and 650cHS4-insulated FV vectors were both significantly less genotoxic than gammaretroviral vectors. Integration sites were evaluated in cord blood CD34(+) cells and the 650cHS4-insulated FV vector had fewer hotspots compared with an uninsulated FV vector. These data suggest that insulated FV vectors are promising for hematopoietic stem cell gene therapy.
Figures



References
-
- Bordignon C, Notarangelo LD, Nobili N, et al. . Gene therapy in peripheral blood lymphocytes and bone marrow for ADA-immunodeficient patients. Science 1995;270:470–475 - PubMed
-
- Hacein-Bey-Abina S, Le Deist F, Carlier F, et al. . Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med 2002;346:1185–1193 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

