Lateral Chain Length in Polyalkyl Acrylates Determines the Mobility of Fibronectin at the Cell/Material Interface
- PMID: 26715432
- PMCID: PMC4732669
- DOI: 10.1021/acs.langmuir.5b03259
Lateral Chain Length in Polyalkyl Acrylates Determines the Mobility of Fibronectin at the Cell/Material Interface
Abstract
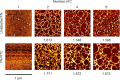
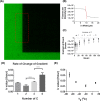
Cells, by interacting with surfaces indirectly through a layer of extracellular matrix proteins, can respond to a variety of physical properties, such as topography or stiffness. Polymer surface mobility is another physical property that is less well understood but has been indicated to hold the potential to modulate cell behavior. Polymer mobility is related to the glass-transition temperature (Tg) of the system, the point at which a polymer transitions from an amorphous solid to a more liquid-like state. This work shows that changes in polymer mobility translate to interfacial mobility of extracellular matrix proteins adsorbed on the material surface. This study has utilized a family of polyalkyl acrylates with similar chemistry but different degrees of mobility, obtained through increasing length of the side chain. These materials are used, in conjunction with fluorescent fibronectin, to determine the mobility of this interfacial layer of protein that constitutes the initial cell-material interface. Furthermore, the extent of fibronectin domain availability (III9, III10, - the integrin binding site), cell-mediated reorganization, and cell differentiation was also determined. A nonmonotonic dependence of fibronectin mobility on polymer surface mobility was observed, with a similar trend noted in cell-mediated reorganization of the protein layer by L929 fibroblasts. The availability of the integrin-binding site was higher on the more mobile surfaces, where a similar organization of the protein into networks at the material interface was observed. Finally, differentiation of C2C12 myoblasts was seen to be highly sensitive to surface mobility upon inhibition of cell contractility. Altogether, these findings show that polymer mobility is a subtle influence that translates to the cell/material interface through the protein layer to alter the biological activity of the surface.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


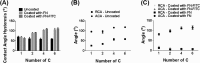



Similar articles
-
The strength of the protein-material interaction determines cell fate.Acta Biomater. 2018 Sep 1;77:74-84. doi: 10.1016/j.actbio.2018.07.016. Epub 2018 Jul 10. Acta Biomater. 2018. PMID: 30006313
-
Myoblast proliferation and differentiation on fibronectin-coated self assembled monolayers presenting different surface chemistries.Biomaterials. 2005 Aug;26(22):4523-31. doi: 10.1016/j.biomaterials.2004.11.028. Epub 2004 Dec 25. Biomaterials. 2005. PMID: 15722121
-
Polymer chain flexibility-induced differences in fetuin A adsorption and its implications on cell attachment and proliferation.Acta Biomater. 2016 Feb;31:89-98. doi: 10.1016/j.actbio.2015.11.039. Epub 2015 Nov 25. Acta Biomater. 2016. PMID: 26607770
-
Integrins in cell adhesion and signaling.Hum Cell. 1996 Sep;9(3):181-6. Hum Cell. 1996. PMID: 9183647 Review.
-
The role of integrin binding sites in fibronectin matrix assembly in vivo.Curr Opin Cell Biol. 2008 Oct;20(5):502-7. doi: 10.1016/j.ceb.2008.06.001. Epub 2008 Jul 21. Curr Opin Cell Biol. 2008. PMID: 18586094 Review.
Cited by
-
Nanoscale Coatings for Ultralow Dose BMP-2-Driven Regeneration of Critical-Sized Bone Defects.Adv Sci (Weinh). 2018 Nov 19;6(2):1800361. doi: 10.1002/advs.201800361. eCollection 2019 Jan 23. Adv Sci (Weinh). 2018. PMID: 30693176 Free PMC article.
-
Role of chemical crosslinking in material-driven assembly of fibronectin (nano)networks: 2D surfaces and 3D scaffolds.Colloids Surf B Biointerfaces. 2016 Dec 1;148:324-332. doi: 10.1016/j.colsurfb.2016.08.044. Epub 2016 Aug 31. Colloids Surf B Biointerfaces. 2016. PMID: 27619185 Free PMC article.
-
Morphological and Surface Potential Characterization of Protein Nanobiofilm Formation on Magnesium Alloy Oxide: Their Role in Biodegradation.Langmuir. 2022 Sep 6;38(35):10854-10866. doi: 10.1021/acs.langmuir.2c01540. Epub 2022 Aug 22. Langmuir. 2022. PMID: 35994730 Free PMC article.
-
Vitronectin as a Micromanager of Cell Response in Material-Driven Fibronectin Nanonetworks.Adv Biosyst. 2017 Sep;1(9):1700047. doi: 10.1002/adbi.201700047. Epub 2017 Aug 10. Adv Biosyst. 2017. PMID: 29497701 Free PMC article.
-
Tailoring Nano-Porous Surface of Aligned Electrospun Poly (L-Lactic Acid) Fibers for Nerve Tissue Engineering.Int J Mol Sci. 2021 Mar 29;22(7):3536. doi: 10.3390/ijms22073536. Int J Mol Sci. 2021. PMID: 33805568 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

