Hif1a Deletion Reveals Pro-Neoplastic Function of B Cells in Pancreatic Neoplasia
- PMID: 26715642
- PMCID: PMC4783189
- DOI: 10.1158/2159-8290.CD-15-0822
Hif1a Deletion Reveals Pro-Neoplastic Function of B Cells in Pancreatic Neoplasia
Abstract
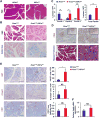
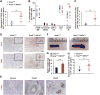
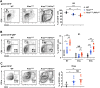
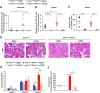
Pancreatic ductal adenocarcinoma (PDAC) is a leading cause of cancer-related deaths worldwide, with an exceedingly low 5-year survival rate. PDAC tumors are characterized by an extensive desmoplastic stromal response and hypovascularity, suggesting that tumor hypoxia could regulate PDAC initiation and/or progression. Using a well-defined, autochthonous Kras(G12D)-driven murine model, as well as human tumors, we demonstrate that hypoxia and stabilization of hypoxia-inducible factor 1α (HIF1α), a principal mediator of hypoxic adaptation, emerge early during preinvasive stages of PDAC. Surprisingly, pancreas-specific Hif1a deletion drastically accelerated Kras(G12D)-driven pancreatic neoplasia and was accompanied by significant increases in intrapancreatic B lymphocytes, featuring prominent influx of a rare "B1b" B-cell subtype. Finally, treatment of HIF1α-deficient mice with B cell-depleting αCD20 monoclonal antibodies inhibited progression of pancreatic intraepithelial neoplasia (PanIN). Our data reveal a previously unrecognized role for B cells in promoting pancreatic tumorigenesis and implicate HIF1α as a critical regulator of PDAC development.
Significance: We show here that pancreas-specific Hif1a deletion promotes PDAC initiation, coincident with increased intrapancreatic accumulation of B cells, and that B-cell depletion suppresses pancreatic tumorigenesis. We therefore demonstrate a protective role for HIF1α in pancreatic cancer initiation and uncover a previously unrecognized function of B cells.
©2015 American Association for Cancer Research.
Conflict of interest statement
Figures

Comment in
-
B Cells Promote Pancreatic Tumorigenesis.Cancer Discov. 2016 Mar;6(3):230-2. doi: 10.1158/2159-8290.CD-16-0100. Cancer Discov. 2016. PMID: 26951836
References
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64:9–29. - PubMed
-
- Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer cell. 2003;4:437–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

