Redundant Regulation of Cdk1 Tyrosine Dephosphorylation in Saccharomyces cerevisiae
- PMID: 26715668
- PMCID: PMC4788127
- DOI: 10.1534/genetics.115.182469
Redundant Regulation of Cdk1 Tyrosine Dephosphorylation in Saccharomyces cerevisiae
Abstract
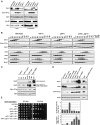
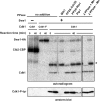
Cdk1 activity drives both mitotic entry and the metaphase-to-anaphase transition in all eukaryotes. The kinase Wee1 and the phosphatase Cdc25 regulate the mitotic activity of Cdk1 by the reversible phosphorylation of a conserved tyrosine residue. Mutation of cdc25 in Schizosaccharomyces pombe blocks Cdk1 dephosphorylation and causes cell cycle arrest. In contrast, deletion of MIH1, the cdc25 homolog in Saccharomyces cerevisiae, is viable. Although Cdk1-Y19 phosphorylation is elevated during mitosis in mih1∆ cells, Cdk1 is dephosphorylated as cells progress into G1, suggesting that additional phosphatases regulate Cdk1 dephosphorylation. Here we show that the phosphatase Ptp1 also regulates Cdk1 dephosphorylation in vivo and can directly dephosphorylate Cdk1 in vitro. Using a novel in vivo phosphatase assay, we also show that PP2A bound to Rts1, the budding yeast B56-regulatory subunit, regulates dephosphorylation of Cdk1 independently of a function regulating Swe1, Mih1, or Ptp1, suggesting that PP2A(Rts1) either directly dephosphorylates Cdk1-Y19 or regulates an unidentified phosphatase.
Keywords: Cdc25/Mih1; Cdk1; PP2A; Wee1/Swe1; mitosis.
Copyright © 2016 by the Genetics Society of America.
Figures

References
-
- Cayla X., Goris J., Hermann J., Hendrix P., Ozon R., et al. , 1990. Isolation and characterization of a tyrosyl phosphatase activator from rabbit skeletal muscle and Xenopus laevis oocytes. Biochemistry 29: 658–667. - PubMed
-
- Dunphy W. G., Kumagai A., 1991. The cdc25 protein contains an intrinsic phosphatase activity. Cell 67: 189–196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

