Mitoguardin-1 and -2 promote maturation and the developmental potential of mouse oocytes by maintaining mitochondrial dynamics and functions
- PMID: 26716412
- PMCID: PMC4811450
- DOI: 10.18632/oncotarget.6713
Mitoguardin-1 and -2 promote maturation and the developmental potential of mouse oocytes by maintaining mitochondrial dynamics and functions
Abstract
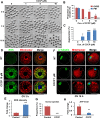
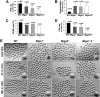
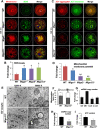
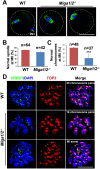
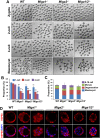
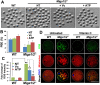
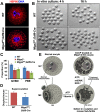
Mitochondrial dynamics change mitochondrial morphological features and numbers as a part of adaptive cellular metabolism, which is vital for most eukaryotic cells and organisms. A disease or even death of an animal can occur if these dynamics are disrupted. Using large-scale genetic screening in fruit flies, we previously found the gene mitoguardin (Miga), which encodes a mitochondrial outer-membrane protein and promotes mitochondrial fusion. Knockout mouse strains were generated for the mammalian Miga homologs Miga1 and Miga2. Miga1/2-/- females show greatly reduced quality of oocytes and early embryos and are subfertile. Mitochondria became clustered in the cytoplasm of oocytes from the germinal-vesicle stage to meiosis II; production of reactive oxygen species increased in mitochondria and caused damage to mitochondrial ultrastructures. Additionally, reduced ATP production, a decreased mitochondrial-DNA copy number, and lower mitochondrial membrane potential were detected in Miga1/2-/- oocytes during meiotic maturation. These changes resulted in low rates of polar-body extrusion during oocyte maturation, reduced developmental potential of the resulting early embryos, and consequently female subfertility. We provide direct evidence that MIGA1/2-regulated mitochondrial dynamics is crucial for mitochondrial functions, ensure oocyte maturation, and maintain the developmental potential.
Keywords: Pathology Section; ROS; female infertility; mitochondrion; mtDNA copy number; oocyte meiosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Mitochondrial Function Regulated by Mitoguardin-1/2 Is Crucial for Ovarian Endocrine Functions and Ovulation.Endocrinology. 2017 Nov 1;158(11):3988-3999. doi: 10.1210/en.2017-00487. Endocrinology. 2017. PMID: 28938432
-
The importance of mitochondrial metabolic activity and mitochondrial DNA replication during oocyte maturation in vitro on oocyte quality and subsequent embryo developmental competence.Mol Reprod Dev. 2012 Jun;79(6):392-401. doi: 10.1002/mrd.22042. Epub 2012 Apr 16. Mol Reprod Dev. 2012. PMID: 22467220
-
Mitoguardin Regulates Mitochondrial Fusion through MitoPLD and Is Required for Neuronal Homeostasis.Mol Cell. 2016 Jan 7;61(1):111-24. doi: 10.1016/j.molcel.2015.11.017. Epub 2015 Dec 17. Mol Cell. 2016. PMID: 26711011
-
Mitochondrial function in the human oocyte and embryo and their role in developmental competence.Mitochondrion. 2011 Sep;11(5):797-813. doi: 10.1016/j.mito.2010.09.012. Epub 2010 Oct 7. Mitochondrion. 2011. PMID: 20933103 Review.
-
[Mitochondrial and oocyte development].Yi Chuan. 2007 Dec;29(12):1429-33. Yi Chuan. 2007. PMID: 18065375 Review. Chinese.
Cited by
-
Uncovering the important role of mitochondrial dynamics in oogenesis: impact on fertility and metabolic disorder transmission.Biophys Rev. 2021 Nov 23;13(6):967-981. doi: 10.1007/s12551-021-00891-w. eCollection 2021 Dec. Biophys Rev. 2021. PMID: 35059021 Free PMC article. Review.
-
Noninvasive metabolic profiling of cumulus cells, oocytes, and embryos via fluorescence lifetime imaging microscopy: a mini-review.Hum Reprod. 2023 May 2;38(5):799-810. doi: 10.1093/humrep/dead063. Hum Reprod. 2023. PMID: 37015098 Free PMC article. Review.
-
The Role of PEDF in Reproductive Aging of the Ovary.Int J Mol Sci. 2022 Sep 8;23(18):10359. doi: 10.3390/ijms231810359. Int J Mol Sci. 2022. PMID: 36142276 Free PMC article.
-
Nicotinamide Mononucleotide and Nicotinamide Riboside Reverse Ovarian Aging in Rats Via Rebalancing Mitochondrial Fission and Fusion Mechanisms.Pharm Res. 2024 May;41(5):921-935. doi: 10.1007/s11095-024-03704-3. Epub 2024 Apr 29. Pharm Res. 2024. PMID: 38684562 Free PMC article.
-
Mitochondrial deoxyguanosine kinase is required for female fertility in mice.Acta Biochim Biophys Sin (Shanghai). 2024 Mar 25;56(3):427-439. doi: 10.3724/abbs.2024003. Acta Biochim Biophys Sin (Shanghai). 2024. PMID: 38327186 Free PMC article.
References
-
- Kogo N, Tazaki A, Kashino Y, Morichika K, Orii H, Mochii M, Watanabe K. Germ-line mitochondria exhibit suppressed respiratory activity to support their accurate transmission to the next generation. Developmental Biology. 2011;349:462–469. - PubMed
-
- Ge H, Tollner TL, Hu Z, Dai M, Li X, Guan H, Shan D, Zhang X, Lv J, Huang C, Dong Q. The importance of mitochondrial metabolic activity and mitochondrial DNA replication during oocyte maturation in vitro on oocyte quality and subsequent embryo developmental competence. Molecular Reproduction and Development. 2012;79:392–401. - PubMed
-
- Cardone L, de Cristofaro T, Affaitati A, Garbi C, Ginsberg MD, Saviano M, Varrone S, Rubin CS, Gottesman ME, Avvedimento EV, Feliciello A. A-kinase anchor protein 84/121 are targeted to mitochondria and mitotic spindles by overlapping amino-terminal motifs. Journal of Molecular Biology. 2002;320:663–675. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

