Autophagy is involved in TGF-β1-induced protective mechanisms and formation of cancer-associated fibroblasts phenotype in tumor microenvironment
- PMID: 26716641
- PMCID: PMC4826194
- DOI: 10.18632/oncotarget.6702
Autophagy is involved in TGF-β1-induced protective mechanisms and formation of cancer-associated fibroblasts phenotype in tumor microenvironment
Abstract
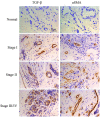
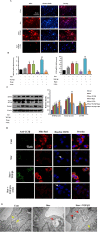
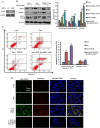
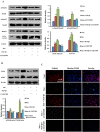
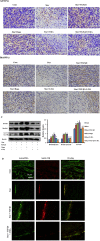
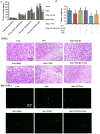
Transforming growth factor-β1 (TGF-β1) present in tumor microenvironment acts in a coordinated fashion to either suppress or promote tumor development. However, the molecular mechanisms underlying the effects of TGF-β1 on tumor microenvironment are not well understood. Our clinical data showed a positive association between TGF-β1 expression and cancer-associated fibroblasts (CAFs) in tumor microenvironment of breast cancer patients. Thus we employed starved NIH3T3 fibroblasts in vitro and 4T1 cells mixed with NIH3T3 fibroblasts xenograft model in vivo to simulate nutritional deprivation of tumor microenvironment to explore the effects of TGF-β1. We demonstrated that TGF-β1 protected NIH3T3 fibroblasts from Star-induced growth inhibition, mitochondrial damage and cell apoptosis. Interestingly, TGF-β1 induced the formation of CAFs phenotype in starvation (Star)-treated NIH3T3 fibroblasts and xenografted Balb/c mice, which promoted breast cancer tumor growth. In both models, autophagy agonist rapamycin increased TGF-β1-induced protective effects and formation of CAFs phenotypes, while autophagy inhibitor 3-methyladenine, Atg5 knockdown or TGF-β type I receptor kinase inhibitor LY-2157299 blocked TGF-β1 induced these effects. Taken together, our results indicated that TGF-β/Smad autophagy was involved in TGF-β1-induced protective effects and formation of CAFs phenotype in tumor microenvironment, which may be used as therapy targets in breast cancer.
Keywords: TGF-β1; autophagy; cancer-associated fibroblasts; mitochondria; tumor microenvironment.
Conflict of interest statement
The authors declare no financial or commercial conflict of interest.
Figures


Similar articles
-
TGF-β1-activated cancer-associated fibroblasts promote breast cancer invasion, metastasis and epithelial-mesenchymal transition by autophagy or overexpression of FAP-α.Biochem Pharmacol. 2021 Jun;188:114527. doi: 10.1016/j.bcp.2021.114527. Epub 2021 Mar 17. Biochem Pharmacol. 2021. PMID: 33741330
-
Osteopontin mediates an MZF1-TGF-β1-dependent transformation of mesenchymal stem cells into cancer-associated fibroblasts in breast cancer.Oncogene. 2015 Sep 10;34(37):4821-33. doi: 10.1038/onc.2014.410. Epub 2014 Dec 22. Oncogene. 2015. PMID: 25531323 Free PMC article.
-
TGF-β1 induces PGP9.5 expression in CAFs to promote the growth of colorectal cancer cells.Oncol Rep. 2017 Jan;37(1):115-122. doi: 10.3892/or.2016.5238. Epub 2016 Nov 9. Oncol Rep. 2017. PMID: 27840994
-
[TGF-β signaling pathways in cancers].Postepy Hig Med Dosw (Online). 2013 Sep 25;67:1008-17. doi: 10.5604/17322693.1068073. Postepy Hig Med Dosw (Online). 2013. PMID: 24088545 Review. Polish.
-
The "Self-eating" of cancer-associated fibroblast: A potential target for cancer.Biomed Pharmacother. 2023 Jul;163:114762. doi: 10.1016/j.biopha.2023.114762. Epub 2023 Apr 24. Biomed Pharmacother. 2023. PMID: 37100015 Review.
Cited by
-
The role of tumor microenvironment on cancer stem cell fate in solid tumors.Cell Commun Signal. 2023 Jun 16;21(1):143. doi: 10.1186/s12964-023-01129-w. Cell Commun Signal. 2023. PMID: 37328876 Free PMC article. Review.
-
Reciprocal Regulation of Cancer-Associated Fibroblasts and Tumor Microenvironment in Gastrointestinal Cancer: Implications for Cancer Dormancy.Cancers (Basel). 2023 Apr 27;15(9):2513. doi: 10.3390/cancers15092513. Cancers (Basel). 2023. PMID: 37173977 Free PMC article. Review.
-
Mapping the Metabolic Networks of Tumor Cells and Cancer-Associated Fibroblasts.Cells. 2021 Feb 2;10(2):304. doi: 10.3390/cells10020304. Cells. 2021. PMID: 33540679 Free PMC article. Review.
-
miR-26 Induces Apoptosis and Inhibits Autophagy in Non-small Cell Lung Cancer Cells by Suppressing TGF-β1-JNK Signaling Pathway.Front Pharmacol. 2019 Jan 9;9:1509. doi: 10.3389/fphar.2018.01509. eCollection 2018. Front Pharmacol. 2019. PMID: 30687089 Free PMC article.
-
A microfluidic chip for screening individual cancer cells via eavesdropping on autophagy-inducing crosstalk in the stroma niche.Sci Rep. 2017 May 17;7(1):2050. doi: 10.1038/s41598-017-02172-7. Sci Rep. 2017. PMID: 28515430 Free PMC article.
References
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
-
- Li T, Yang Y, Hua X, Wang G, Liu W, Jia C, Tai Y, Zhang Q, Chen G. Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer letters. 2012;318:154–161. - PubMed
-
- Chen H, Yang WW, Wen QT, Xu L, Chen M. TGF-beta induces fibroblast activation protein expression; fibroblast activation protein expression increases the proliferation, adhesion, and migration of HO-8910PM [corrected] Experimental and molecular pathology. 2009;87:189–194. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

