A Single Amino Acid Deletion (ΔF1502) in the S6 Segment of CaV2.1 Domain III Associated with Congenital Ataxia Increases Channel Activity and Promotes Ca2+ Influx
- PMID: 26716990
- PMCID: PMC4696675
- DOI: 10.1371/journal.pone.0146035
A Single Amino Acid Deletion (ΔF1502) in the S6 Segment of CaV2.1 Domain III Associated with Congenital Ataxia Increases Channel Activity and Promotes Ca2+ Influx
Abstract
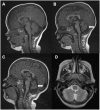
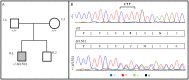
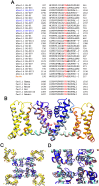
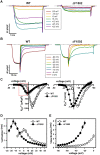
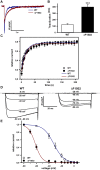
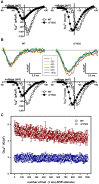
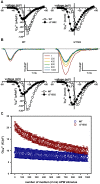
Mutations in the CACNA1A gene, encoding the pore-forming CaV2.1 (P/Q-type) channel α1A subunit, result in heterogeneous human neurological disorders, including familial and sporadic hemiplegic migraine along with episodic and progressive forms of ataxia. Hemiplegic Migraine (HM) mutations induce gain-of-channel function, mainly by shifting channel activation to lower voltages, whereas ataxia mutations mostly produce loss-of-channel function. However, some HM-linked gain-of-function mutations are also associated to congenital ataxia and/or cerebellar atrophy, including the deletion of a highly conserved phenylalanine located at the S6 pore region of α1A domain III (ΔF1502). Functional studies of ΔF1502 CaV2.1 channels, expressed in Xenopus oocytes, using the non-physiological Ba2+ as the charge carrier have only revealed discrete alterations in channel function of unclear pathophysiological relevance. Here, we report a second case of congenital ataxia linked to the ΔF1502 α1A mutation, detected by whole-exome sequencing, and analyze its functional consequences on CaV2.1 human channels heterologously expressed in mammalian tsA-201 HEK cells, using the physiological permeant ion Ca2+. ΔF1502 strongly decreases the voltage threshold for channel activation (by ~ 21 mV), allowing significantly higher Ca2+ current densities in a range of depolarized voltages with physiological relevance in neurons, even though maximal Ca2+ current density through ΔF1502 CaV2.1 channels is 60% lower than through wild-type channels. ΔF1502 accelerates activation kinetics and slows deactivation kinetics of CaV2.1 within a wide range of voltage depolarization. ΔF1502 also slowed CaV2.1 inactivation kinetic and shifted the inactivation curve to hyperpolarized potentials (by ~ 28 mV). ΔF1502 effects on CaV2.1 activation and deactivation properties seem to be of high physiological relevance. Thus, ΔF1502 strongly promotes Ca2+ influx in response to either single or trains of action potential-like waveforms of different durations. Our observations support a causative role of gain-of-function CaV2.1 mutations in congenital ataxia, a neurodevelopmental disorder at the severe-most end of CACNA1A-associated phenotypic spectrum.
Conflict of interest statement
Figures

References
-
- Pineda JC, Waters RS, Foehring RC. Specificity in the interaction of HVA Ca2+ channel types with Ca2+-dependent AHPs and firing behavior in neocortical pyramidal neurons. J Neurophysiol. 1998;79: 2522–2534. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

