Development of sigma-1 (σ1) receptor fluorescent ligands as versatile tools to study σ1 receptors
- PMID: 26717207
- PMCID: PMC4755300
- DOI: 10.1016/j.ejmech.2015.12.014
Development of sigma-1 (σ1) receptor fluorescent ligands as versatile tools to study σ1 receptors
Abstract
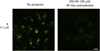
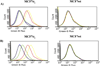
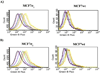
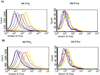
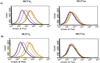
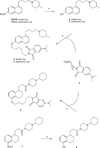
Despite their controversial physiology, sigma-1 (σ1) receptors are intriguing targets for the development of therapeutic agents for central nervous system diseases. With the aim of providing versatile pharmacological tools to study σ1 receptors, we developed three σ1 fluorescent tracers by functionalizing three well characterized σ1 ligands with a fluorescent tag. A good compromise between σ1 binding affinity and fluorescent properties was reached, and the σ1 specific targeting of the novel tracers was demonstrated by confocal microscopy and flow cytometry. These novel ligands were also successfully used in competition binding studies by flow cytometry, showing their utility in nonradioactive binding assays as an alternative strategy to the more classical radioligand binding assays. To the best of our knowledge these are the first σ1 fluorescent ligands to be developed and successfully employed in living cells, representing promising tools to strengthen σ1 receptors related studies.
Keywords: Fluorescent ligand; Sigma receptors.
Copyright © 2015 Elsevier Masson SAS. All rights reserved.
Figures

Similar articles
-
Novel and Selective Fluorescent σ2 -Receptor Ligand with a 3,4-Dihydroisoquinolin-1-one Scaffold: A Tool to Study σ2 Receptors in Living Cells.Chembiochem. 2015 May 4;16(7):1078-83. doi: 10.1002/cbic.201402712. Epub 2015 Mar 10. Chembiochem. 2015. PMID: 25757101
-
Novel derivatives of 1-cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydronaphthalen-1-yl)propyl]piperazine (PB28) with improved fluorescent and σ receptors binding properties.J Med Chem. 2014 Apr 24;57(8):3314-23. doi: 10.1021/jm401874n. Epub 2014 Apr 14. J Med Chem. 2014. PMID: 24697311
-
Quantum Dot Based Luminescent Nanoprobes for Sigma-2 Receptor Imaging.Mol Pharm. 2018 Feb 5;15(2):458-471. doi: 10.1021/acs.molpharmaceut.7b00825. Epub 2017 Dec 22. Mol Pharm. 2018. PMID: 29226684
-
Medicinal Chemistry of σ1 Receptor Ligands: Pharmacophore Models, Synthesis, Structure Affinity Relationships, and Pharmacological Applications.Handb Exp Pharmacol. 2017;244:51-79. doi: 10.1007/164_2017_33. Handb Exp Pharmacol. 2017. PMID: 28620761 Review.
-
Multi-Target Directed Ligands (MTDLs) Binding the σ1 Receptor as Promising Therapeutics: State of the Art and Perspectives.Int J Mol Sci. 2021 Jun 14;22(12):6359. doi: 10.3390/ijms22126359. Int J Mol Sci. 2021. PMID: 34198620 Free PMC article. Review.
Cited by
-
Development of Fluorescent 4-[4-(3H-Spiro[isobenzofuran-1,4'-piperidin]-1'-yl)butyl]indolyl Derivatives as High-Affinity Probes to Enable the Study of σ Receptors via Fluorescence-Based Techniques.J Med Chem. 2023 Mar 23;66(6):3798-3817. doi: 10.1021/acs.jmedchem.2c01227. Epub 2023 Mar 15. J Med Chem. 2023. PMID: 36919956 Free PMC article.
-
SIGMAP: an explainable artificial intelligence tool for SIGMA-1 receptor affinity prediction.RSC Med Chem. 2024 Nov 8;16(2):835-848. doi: 10.1039/d4md00722k. eCollection 2025 Feb 19. RSC Med Chem. 2024. PMID: 39618965 Free PMC article.
-
Intracellular dynamics of the Sigma-1 receptor observed with super-resolution imaging microscopy.PLoS One. 2022 May 18;17(5):e0268563. doi: 10.1371/journal.pone.0268563. eCollection 2022. PLoS One. 2022. PMID: 35584184 Free PMC article.
-
Multifunctional thiosemicarbazones and deconstructed analogues as a strategy to study the involvement of metal chelation, Sigma-2 (σ2) receptor and P-gp protein in the cytotoxic action: In vitro and in vivo activity in pancreatic tumors.Eur J Med Chem. 2018 Jan 20;144:359-371. doi: 10.1016/j.ejmech.2017.12.024. Epub 2017 Dec 8. Eur J Med Chem. 2018. PMID: 29287249 Free PMC article.
-
PB28, the Sigma-1 and Sigma-2 Receptors Modulator With Potent Anti-SARS-CoV-2 Activity: A Review About Its Pharmacological Properties and Structure Affinity Relationships.Front Pharmacol. 2020 Dec 7;11:589810. doi: 10.3389/fphar.2020.589810. eCollection 2020. Front Pharmacol. 2020. PMID: 33364961 Free PMC article. Review.
References
-
- Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effect of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 1976;197:517–532. - PubMed
-
- Quirion R, Bowen WD, Itzhak Y, Junien JL, Musacchio JM, Rothman RB, Su TP, Tam SW, Taylor DP. A proposal for the classification of sigma binding sites. Trends Pharmacol. Sci. 1992;13:85–86. - PubMed
-
- Colabufo NA, Berardi F, Abate C, Contino M, Niso M, Perrone R. Is the σ2 receptor a histone binding protein? J. Med. Chem. 2006;49:4153–4158. - PubMed
-
- Abate C, Niso M, Infantino V, Menga A, Berardi F. Elements in support of the ‘non-identity’ of the PGRMC1 protein with the σ2 receptor. Eur. J. Pharmacol. 2015;758:16–23. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

