Development of sigma-1 (σ1) receptor fluorescent ligands as versatile tools to study σ1 receptors
- PMID: 26717207
- PMCID: PMC4755300
- DOI: 10.1016/j.ejmech.2015.12.014
Development of sigma-1 (σ1) receptor fluorescent ligands as versatile tools to study σ1 receptors
Abstract
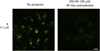
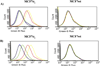
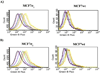
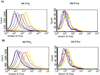
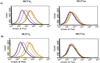
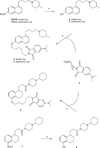
Despite their controversial physiology, sigma-1 (σ1) receptors are intriguing targets for the development of therapeutic agents for central nervous system diseases. With the aim of providing versatile pharmacological tools to study σ1 receptors, we developed three σ1 fluorescent tracers by functionalizing three well characterized σ1 ligands with a fluorescent tag. A good compromise between σ1 binding affinity and fluorescent properties was reached, and the σ1 specific targeting of the novel tracers was demonstrated by confocal microscopy and flow cytometry. These novel ligands were also successfully used in competition binding studies by flow cytometry, showing their utility in nonradioactive binding assays as an alternative strategy to the more classical radioligand binding assays. To the best of our knowledge these are the first σ1 fluorescent ligands to be developed and successfully employed in living cells, representing promising tools to strengthen σ1 receptors related studies.
Keywords: Fluorescent ligand; Sigma receptors.
Copyright © 2015 Elsevier Masson SAS. All rights reserved.
Figures

References
-
- Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effect of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 1976;197:517–532. - PubMed
-
- Quirion R, Bowen WD, Itzhak Y, Junien JL, Musacchio JM, Rothman RB, Su TP, Tam SW, Taylor DP. A proposal for the classification of sigma binding sites. Trends Pharmacol. Sci. 1992;13:85–86. - PubMed
-
- Colabufo NA, Berardi F, Abate C, Contino M, Niso M, Perrone R. Is the σ2 receptor a histone binding protein? J. Med. Chem. 2006;49:4153–4158. - PubMed
-
- Abate C, Niso M, Infantino V, Menga A, Berardi F. Elements in support of the ‘non-identity’ of the PGRMC1 protein with the σ2 receptor. Eur. J. Pharmacol. 2015;758:16–23. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

