Investigating the position of the hairpin loop in New Delhi metallo-β-lactamase, NDM-1, during catalysis and inhibitor binding
- PMID: 26717260
- PMCID: PMC4843777
- DOI: 10.1016/j.jinorgbio.2015.10.011
Investigating the position of the hairpin loop in New Delhi metallo-β-lactamase, NDM-1, during catalysis and inhibitor binding
Abstract
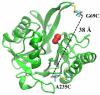
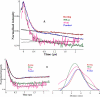
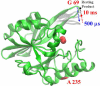
In an effort to examine the relative position of a hairpin loop in New Delhi metallo-β-lactamase, NDM-1, during catalysis, rapid freeze quench double electron electron resonance (RFQ-DEER) spectroscopy was used. A doubly-labeled mutant of NDM-1, which had one spin label on the invariant loop at position 69 and another label at position 235, was prepared and characterized. The reaction of the doubly spin labeled mutant with chromacef was freeze quenched at 500μs and 10ms. DEER results showed that the average distance between labels decreased by 4Å in the 500μs quenched sample and by 2Å in the 10ms quenched sample, as compared to the distance in the unreacted enzyme, although the peaks corresponding to distance distributions were very broad. DEER spectra with the doubly spin labeled enzyme with two inhibitors showed that the distance between the loop residue at position 69 and the spin label at position 235 does not change upon inhibitor binding. This study suggests that the hairpin loop in NDM-1 moves over the metal ion during the catalysis and then moves back to its original position after hydrolysis, which is consistent with a previous hypothesis based on NMR solution studies on a related metallo-β-lactamase. This study also demonstrates that this loop motion occurs in the millisecond time domain.
Keywords: DEER spectroscopy; Metallo-β-lactamase; Rapid freeze quench; Site specific spin labeling.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

References
-
- O'Neill J. AMA Review on Antimocrobial Resistance by Untied Kingdom Government. 2014.
-
- Antibiotic Resistance Threat Repost, CDC Eurosurveillance. 2013;18:28–28.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

