The Development of Macrophage-Mediated Cell Therapy to Improve Skeletal Muscle Function after Injury
- PMID: 26717325
- PMCID: PMC4696731
- DOI: 10.1371/journal.pone.0145550
The Development of Macrophage-Mediated Cell Therapy to Improve Skeletal Muscle Function after Injury
Abstract
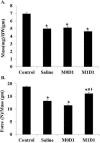
Skeletal muscle regeneration following acute injury is a multi-step process involving complex changes in tissue microenvironment. Macrophages (MPs) are one of the key cell types involved in orchestration and modulation of the repair process. Multiple studies highlight the essential role of MPs in the control of the myogenic program and inflammatory response during skeletal muscle regeneration. A variety of MP phenotypes have been identified and characterized in vitro as well as in vivo. As such, MPs hold great promise for cell-based therapies in the field of regenerative medicine. In this study we used bone-marrow derived in vitro LPS/IFN-y-induced M1 MPs to enhance functional muscle recovery after tourniquet-induced ischemia/reperfusion injury (TK-I/R). We detected a 15% improvement in specific tension and force normalized to mass after M1 (LPS/IFN-γ) MP transplantation 24 hours post-reperfusion. Interestingly, we found that M0 bone marrow-derived unpolarized MPs significantly impaired muscle function highlighting the complexity of temporally coordinated skeletal muscle regenerative program. Furthermore, we show that delivery of M1 (LPS/IFN-γ) MPs early in regeneration accelerates myofiber repair, decreases fibrotic tissue deposition and increases whole muscle IGF-I expression.
Conflict of interest statement
Figures







References
-
- Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovascular surgery. 2002;10(6):620–30. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

