Heterozygous Disruption of Autism susceptibility candidate 2 Causes Impaired Emotional Control and Cognitive Memory
- PMID: 26717414
- PMCID: PMC4699902
- DOI: 10.1371/journal.pone.0145979
Heterozygous Disruption of Autism susceptibility candidate 2 Causes Impaired Emotional Control and Cognitive Memory
Abstract
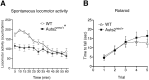
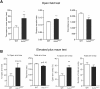
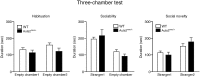
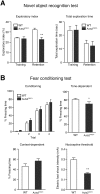
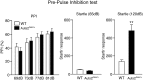
Mutations in the Autism susceptibility candidate 2 gene (AUTS2) have been associated with a broad range of psychiatric illnesses including autism spectrum disorders, intellectual disability and schizophrenia. We previously demonstrated that the cytoplasmic AUTS2 acts as an upstream factor for the Rho family small GTPase Rac1 and Cdc42 that regulate the cytoskeletal rearrangements in neural cells. Moreover, genetic ablation of the Auts2 gene in mice has resulted in defects in neuronal migration and neuritogenesis in the developing cerebral cortex caused by inactivation of Rac1-signaling pathway, suggesting that AUTS2 is required for neural development. In this study, we conducted a battery of behavioral analyses on Auts2 heterozygous mutant mice to examine the involvement of Auts2 in adult cognitive brain functions. Auts2-deficient mice displayed a decrease in exploratory behavior as well as lower anxiety-like behaviors in the absence of any motor dysfunction. Furthermore, the capability for novel object recognition and cued associative memory were impaired in Auts2 mutant mice. Social behavior and sensory motor gating functions were, however, normal in the mutant mice as assessed by the three-chamber test and prepulse inhibition test, respectively. Together, our findings indicate that AUTS2 is critical for the acquisition of neurocognitive function.
Conflict of interest statement
Figures
References
-
- Sultana R, Yu CE, Yu J, Munson J, Chen D, Hua W, et al. Identification of a novel gene on chromosome 7q11.2 interrupted by a translocation breakpoint in a pair of autistic twins. Genomics. 2002;80(2):129–34. . - PubMed
-
- Kalscheuer VM, FitzPatrick D, Tommerup N, Bugge M, Niebuhr E, Neumann LM, et al. Mutations in autism susceptibility candidate 2 (AUTS2) in patients with mental retardation. Hum Genet. 2007;121(3–4):501–9. . - PubMed
-
- Jolley A, Corbett M, McGregor L, Waters W, Brown S, Nicholl J, et al. De novo intragenic deletion of the autism susceptibility candidate 2 (AUTS2) gene in a patient with developmental delay: a case report and literature review. Am J Med Genet A. 2013;161A(6):1508–12. 10.1002/ajmg.a.35922 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

