Two-step ion-exchange chromatographic purification combined with reversed-phase chromatography to isolate C-peptide for mass spectrometric analysis
- PMID: 26717885
- PMCID: PMC6053671
- DOI: 10.1002/jssc.201500989
Two-step ion-exchange chromatographic purification combined with reversed-phase chromatography to isolate C-peptide for mass spectrometric analysis
Abstract
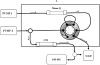
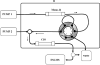
A liquid chromatography with mass spectrometry on-line platform that includes the orthogonal techniques of ion exchange and reversed phase chromatography is applied for C-peptide analysis. Additional improvement is achieved by the subsequent application of cation- and anion-exchange purification steps that allow for isolating components that have their isoelectric points in a narrow pH range before final reversed-phase mass spectrometry analysis. The utility of this approach for isolating fractions in the desired "pI window" for profiling complex mixtures is discussed.
Keywords: C-peptide; Ion exchange chromatography; Isotope dilution assay; Orthogonal separations; Peptide purification.
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures





References
-
- Wagner K, Miliotis T, Marko-Varga G, Bischoff R, Unger KK. Anal. Chem. 2002;74:809–820. - PubMed
-
- Henry R. Principles of multidimensional HPLC and advantages for protein and peptide analysis; Lecture L-5 at 23rd International Symposium on the Separation of Proteins, Peptides and Polynucleotides (ICPPP); November 2003; Delray Beach, FL, USA.
-
- Stoyanov A. Electrophoresis. 2012;33:3281–3290. - PubMed
-
- Kuzuya H, Blix PM, Horwitz DL, Rubinstein AH, Steiner DF, Binder C, Faber OK. Diabetes. 1978;27:184–191. - PubMed
-
- Steiner DF. N. Engl. J. Med. 1969;280:1106–1113. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

