Glucagon-like peptide-1 protects cardiomyocytes from advanced oxidation protein product-induced apoptosis via the PI3K/Akt/Bad signaling pathway
- PMID: 26717963
- PMCID: PMC4732836
- DOI: 10.3892/mmr.2015.4724
Glucagon-like peptide-1 protects cardiomyocytes from advanced oxidation protein product-induced apoptosis via the PI3K/Akt/Bad signaling pathway
Abstract
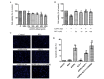
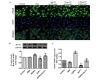
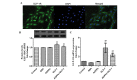
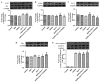
Cardiomyocyte apoptosis is a major event in the pathogenesis of diabetic cardiomyopathy. Currently, no single effective treatment for diabetic cardiomyopathy exists. The present study investigated whether advanced oxidative protein products (AOPPs) have a detrimental role in the survival of cardiomyocytes and if glucagon-like peptide-1 (GLP-1) exerts a cardioprotective effect under these circumstances. The present study also aimed to determine the underlying mechanisms. H9c2 cells were exposed to increasing concentrations of AOPPs in the presence or absence of GLP-1, and the viability and apoptotic rate were detected using a cell counting kit-8 assay and flow cytometry, respectively. In addition, a phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) inhibitor, LY294002, was employed to illustrate the mechanism of the antiapoptotic effect of GLP-1. The expression levels of the apoptotic-associated proteins, Akt, B-cell lymphoma (Bcl)-2, Bcl-2-associated death promoter (Bad), Bcl-2-associated X protein (Bax) and caspase-3 were measured by western blotting. It was revealed that GLP-1 significantly attenuated AOPP-induced cell toxicity and apoptosis. AOPPs inactivated the phosphorylation of Akt, reduced the phosphorylation of Bad, decreased the expression of Bcl-2, increased the expression of Bax and the activation of caspase-3 in H9c2 cells. GLP-1 reversed the above changes induced by AOPPs and the protective effects of GLP-1 were abolished by the PI3K inhibitor, LY294002. In conclusion, the present data suggested that GLP-1 protected cardiomyocytes against AOPP-induced apoptosis, predominantly via the PI3K/Akt/Bad pathway. These results provided a conceivable mechanism for the development of diabetic cardiomyopathy and rendered a novel application of GLP-1 exerting favorable cardiac effects for the treatment of diabetic cardiomyopathy.
Figures
Similar articles
-
Glucagon-like peptide-1 inhibits the receptor for advanced glycation endproducts to prevent podocyte apoptosis induced by advanced oxidative protein products.Biochem Biophys Res Commun. 2017 Jan 22;482(4):1413-1419. doi: 10.1016/j.bbrc.2016.12.050. Epub 2016 Dec 10. Biochem Biophys Res Commun. 2017. PMID: 27965099
-
Glucagon-like peptide-1 attenuates endoplasmic reticulum stress-induced apoptosis in H9c2 cardiomyocytes during hypoxia/reoxygenation through the GLP-1R/PI3K/Akt pathways.Naunyn Schmiedebergs Arch Pharmacol. 2019 Jun;392(6):715-722. doi: 10.1007/s00210-019-01625-2. Epub 2019 Feb 14. Naunyn Schmiedebergs Arch Pharmacol. 2019. PMID: 30762075
-
Geniposide Prevents Hypoxia/Reoxygenation-Induced Apoptosis in H9c2 Cells: Improvement of Mitochondrial Dysfunction and Activation of GLP-1R and the PI3K/AKT Signaling Pathway.Cell Physiol Biochem. 2016;39(1):407-21. doi: 10.1159/000445634. Epub 2016 Jul 4. Cell Physiol Biochem. 2016. PMID: 27372651
-
PI3Ks in Diabetic Cardiomyopathy.J Cardiovasc Pharmacol. 2017 Dec;70(6):422-429. doi: 10.1097/FJC.0000000000000511. J Cardiovasc Pharmacol. 2017. PMID: 28654509 Review.
-
Protective molecular mechanisms of clusterin against apoptosis in cardiomyocytes.Heart Fail Rev. 2018 Jan;23(1):123-129. doi: 10.1007/s10741-017-9654-z. Heart Fail Rev. 2018. PMID: 28948410 Review.
Cited by
-
Multiple Mechanistic Action of Brevinin-1FL Peptide against Oxidative Stress Effects in an Acute Inflammatory Model of Carrageenan-Induced Damage.Oxid Med Cell Longev. 2022 Sep 5;2022:2615178. doi: 10.1155/2022/2615178. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36105482 Free PMC article.
-
All-trans-retinoic acid-mediated cytoprotection in LLC-PK1 renal epithelial cells is coupled to p-ERK activation in a ROS-independent manner.Am J Physiol Renal Physiol. 2017 Dec 1;313(6):F1200-F1208. doi: 10.1152/ajprenal.00085.2017. Epub 2017 Aug 2. Am J Physiol Renal Physiol. 2017. PMID: 28768661 Free PMC article.
-
Glycyrrhetinic acid protects H9c2 cells from oxygen glucose deprivation-induced injury through the PI3K/AKt signaling pathway.J Nat Med. 2017 Jan;71(1):27-35. doi: 10.1007/s11418-016-1023-z. Epub 2016 Jul 12. J Nat Med. 2017. PMID: 27406329
-
Panax notoginseng saponins promote liver regeneration through activation of the PI3K/AKT/mTOR cell proliferation pathway and upregulation of the AKT/Bad cell survival pathway in mice.BMC Complement Altern Med. 2019 Jun 10;19(1):122. doi: 10.1186/s12906-019-2536-2. BMC Complement Altern Med. 2019. PMID: 31182089 Free PMC article.
-
Semaglutide attenuates pathological electrophysiological remodeling in diabetic cardiomyopathy via restoring Cx43 expression.Endocrine. 2024 Jun;84(3):969-979. doi: 10.1007/s12020-024-03823-2. Epub 2024 Apr 22. Endocrine. 2024. PMID: 38647981
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

