Homeostatic responses of colonic LGR5+ stem cells following acute in vivo exposure to a genotoxic carcinogen
- PMID: 26717997
- PMCID: PMC4804129
- DOI: 10.1093/carcin/bgv250
Homeostatic responses of colonic LGR5+ stem cells following acute in vivo exposure to a genotoxic carcinogen
Abstract
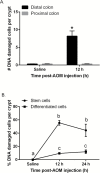
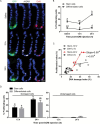
Perturbations in DNA damage, DNA repair, apoptosis and cell proliferation in the base of the crypt where stem cells reside are associated with colorectal cancer (CRC) initiation and progression. Although the transformation of leucine-rich repeat-containing G protein-coupled receptor 5 (Lgr5)(+) cells is an extremely efficient route towards initiating small intestinal adenomas, the role of Lgr5(+) cells in CRC pathogenesis has not been well investigated. Therefore, we further characterized the properties of colonic Lgr5(+) cells compared to differentiated cells in Lgr5-EGFP-IRES-creER(T2) knock-in mice at the initiation stage of carcinogen azoxymethane (AOM)-induced tumorigenesis using a quantitative immunofluorescence microscopy approach. At 12 and 24h post-AOM treatment, colonic Lgr5(+) stem cells (GFP(high)) were preferentially damaged by carcinogen, exhibiting a 4.7-fold induction of apoptosis compared to differentiated (GFP(neg)) cells. Furthermore, with respect to DNA repair, O(6)-methylguanine DNA methyltransferase (MGMT) expression was preferentially induced (by 18.5-fold) in GFP(high) cells at 24h post-AOM treatment compared to GFP(neg) differentiated cells. This corresponded with a 4.3-fold increase in cell proliferation in GFP(high) cells. These data suggest that Lgr5(+) stem cells uniquely respond to alkylation-induced DNA damage by upregulating DNA damage repair, apoptosis and cell proliferation compared to differentiated cells in order to maintain genomic integrity. These findings highlight the mechanisms by which colonic Lgr5(+) stem cells respond to cancer-causing environmental factors.
© The Author 2015. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures



Similar articles
-
Establishment of a multicomponent dietary bioactive human equivalent dose to delete damaged Lgr5+ stem cells using a mouse colon tumor initiation model.Eur J Cancer Prev. 2019 Sep;28(5):383-389. doi: 10.1097/CEJ.0000000000000465. Eur J Cancer Prev. 2019. PMID: 30234553 Free PMC article.
-
Rapidly cycling Lgr5+ stem cells are exquisitely sensitive to extrinsic dietary factors that modulate colon cancer risk.Cell Death Dis. 2016 Nov 10;7(11):e2460. doi: 10.1038/cddis.2016.269. Cell Death Dis. 2016. PMID: 27831561 Free PMC article.
-
Dynamics of Proliferative and Quiescent Stem Cells in Liver Homeostasis and Injury.Gastroenterology. 2017 Oct;153(4):1133-1147. doi: 10.1053/j.gastro.2017.07.006. Epub 2017 Jul 14. Gastroenterology. 2017. PMID: 28716722
-
Environmental Impact on Intestinal Stem Cell Functions in Mucosal Homeostasis and Tumorigenesis.J Cell Biochem. 2017 May;118(5):943-952. doi: 10.1002/jcb.25719. Epub 2017 Jan 11. J Cell Biochem. 2017. PMID: 27584938 Free PMC article. Review.
-
Defining the role of Lgr5+ stem cells in colorectal cancer: from basic research to clinical applications.Genome Med. 2017 Jul 18;9(1):66. doi: 10.1186/s13073-017-0460-y. Genome Med. 2017. PMID: 28720124 Free PMC article. Review.
Cited by
-
Green Tea Leaves and Rosemary Extracts Selectively Induce Cell Death in Triple-Negative Breast Cancer Cells and Cancer Stem Cells and Enhance the Efficacy of Common Chemotherapeutics.Evid Based Complement Alternat Med. 2024 Jan 25;2024:9458716. doi: 10.1155/2024/9458716. eCollection 2024. Evid Based Complement Alternat Med. 2024. PMID: 39376573 Free PMC article.
-
LGR5: An emerging therapeutic target for cancer metastasis and chemotherapy resistance.Cancer Metastasis Rev. 2025 Jan 17;44(1):23. doi: 10.1007/s10555-024-10239-x. Cancer Metastasis Rev. 2025. PMID: 39821694 Free PMC article. Review.
-
Association between red meat consumption and colon cancer: A systematic review of experimental results.Exp Biol Med (Maywood). 2017 Apr;242(8):813-839. doi: 10.1177/1535370217693117. Epub 2017 Jan 1. Exp Biol Med (Maywood). 2017. PMID: 28205448 Free PMC article.
-
Recent Updates on Mechanisms of Resistance to 5-Fluorouracil and Reversal Strategies in Colon Cancer Treatment.Biology (Basel). 2021 Aug 31;10(9):854. doi: 10.3390/biology10090854. Biology (Basel). 2021. PMID: 34571731 Free PMC article. Review.
-
DNA damage in aging, the stem cell perspective.Hum Genet. 2020 Mar;139(3):309-331. doi: 10.1007/s00439-019-02047-z. Epub 2019 Jul 19. Hum Genet. 2020. PMID: 31324975 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

