Bone marrow-derived mesenchymal stem cells rescue injured H9c2 cells via transferring intact mitochondria through tunneling nanotubes in an in vitro simulated ischemia/reperfusion model
- PMID: 26718099
- PMCID: PMC4732861
- DOI: 10.3892/mmr.2015.4726
Bone marrow-derived mesenchymal stem cells rescue injured H9c2 cells via transferring intact mitochondria through tunneling nanotubes in an in vitro simulated ischemia/reperfusion model
Abstract
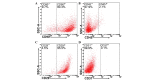
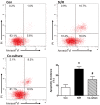
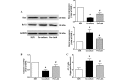
The transplantation of mesenchymal stem cells (MSCs) is considered to be a promising treatment for ischemic heart disease; however, the therapeutic effects and underlying mechanisms of action require further evaluation. Mitochondrial dysfunction is a key event in simulated ischemia/reperfusion (SI/R) injury. The purpose of the present study was to investigate the mechanism of mitochondrial transfer, which may be involved the antiapoptotic action of co-culture with MSCs. An in vitro model of simulated ischemia/reperfusion (SI/R) was used in the present study. The apoptotic indexes were significantly increased when H9c2 cardiomyocytes were induced in the SI/R group. Following co-culture with bone marrow-derived (BM)-MSCs, H9c2 cells exhibited marked resistance against the SI/R-induced apoptotic process. Besides, mitochondrial transfer via a tunneling nanotube (TNT) like structure was detected by confocal fluorescent microscopy. In addition, following pretreated with latrunculin-A (LatA), an inhibitor of TNT formation, the BM-MSCs were not able to rescue injured H9c2 cells from apoptosis, as previously observed. In conclusion, the anti-apoptotic ability of BM-MSCs may be partially attributed to the recovery of mitochondrial dysfunction in SI/R, and the formation of TNTs appears to be involved in this action of mitochondrial transfer between adjacent cells.
Figures



References
-
- Forouzanfar MH, Moran AE, Flaxman AD, Roth G, Mensah GA, Ezzati M, Naghavi M, Murray CJ. Assessing the global burden of ischemic heart disease, part 2: analytic methods and estimates of the global epidemiology of ischemic heart disease in 2010. Glob Heart. 2012;7:331–342. doi: 10.1016/j.gheart.2012.10.003. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

