An Improved Immunostaining and Imaging Methodology to Determine Cell and Protein Distributions within the Bone Environment
- PMID: 26718242
- PMCID: PMC4810797
- DOI: 10.1369/0022155415626765
An Improved Immunostaining and Imaging Methodology to Determine Cell and Protein Distributions within the Bone Environment
Abstract
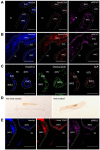
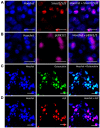
Bone is a dynamic tissue that undergoes multiple changes throughout its lifetime. Its maintenance requires a tight regulation between the cells embedded within the bone matrix, and an imbalance among these cells may lead to bone diseases such as osteoporosis. Identifying cell populations and their proteins within bone is necessary for understanding bone biology. Immunolabeling is one approach used to visualize proteins in tissues. Efficient immunolabeling of bone samples often requires decalcification, which may lead to changes in the structural morphology of the bone. Recently, methyl-methacrylate embedding of non-decalcified tissue followed by heat-induced antigen retrieval has been used to process bone sections for immunolabeling. However, this technique is applicable for bone slices below 50-µm thickness while fixed on slides. Additionally, enhancing epitope exposure for immunolabeling is still a challenge. Moreover, imaging bone cells within the bone environment using standard confocal microscopy is difficult. Here we demonstrate for the first time an improved methodology for immunolabeling non-decalcified bone using a testicular hyaluronidase enzyme-based antigen retrieval technique followed by two-photon fluorescence laser microscopy (TPLM) imaging. This procedure allowed us to image key intracellular proteins in bone cells while preserving the structural morphology of the cells and the bone.
Keywords: bone; confocal microscopy; immunolabeling; osteoblasts; osteocytes; testicular hyaluronidase; two photon fluorescence laser microscopy.
© 2016 The Histochemical Society.
Conflict of interest statement
Figures



References
-
- An YH, Martin KL. (2003). Histological techniques for decalcified bone and cartilage Handbook of Histology Methods for Bone and Cartilage. New York: Humana Press.
-
- Bancroft JD, Stevens A. (1982). Theory and Practice of Histological Techniques, 2nd edition, New York: Churchill Livingstone.
-
- Brandi ML. (2009). Microarchitecture, the key to bone quality. Rheumatology (Oxford) 48 Suppl 4:iv3-8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

