Allogeneic guinea pig mesenchymal stem cells ameliorate neurological changes in experimental colitis
- PMID: 26718461
- PMCID: PMC4697327
- DOI: 10.1186/s13287-015-0254-3
Allogeneic guinea pig mesenchymal stem cells ameliorate neurological changes in experimental colitis
Abstract
Background: The use of mesenchymal stem cells (MSCs) to treat inflammatory bowel disease (IBD) is of great interest because of their immunomodulatory properties. Damage to the enteric nervous system (ENS) is implicated in IBD pathophysiology and disease progression. The most commonly used model to study inflammation-induced changes to the ENS is 2,4,6-trinitrobenzene-sulfonate acid (TNBS)-induced colitis in guinea pigs; however, no studies using guinea pig MSCs in colitis have been performed. This study aims to isolate and characterise guinea pig MSCs and then test their therapeutic potential for the treatment of enteric neuropathy associated with intestinal inflammation.
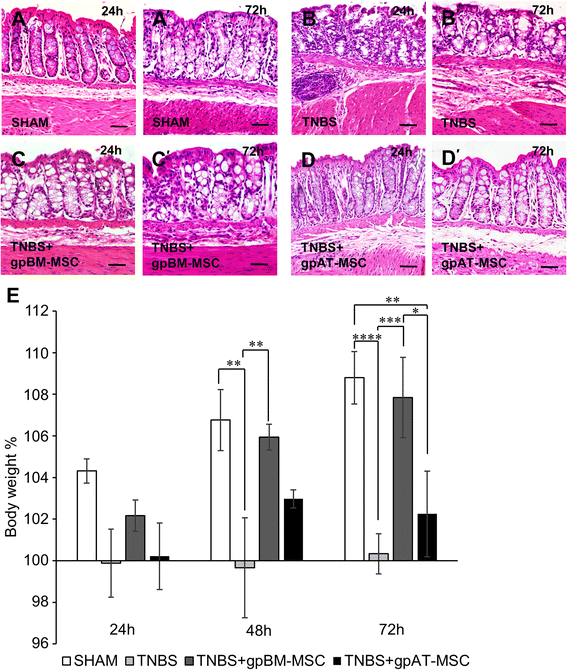
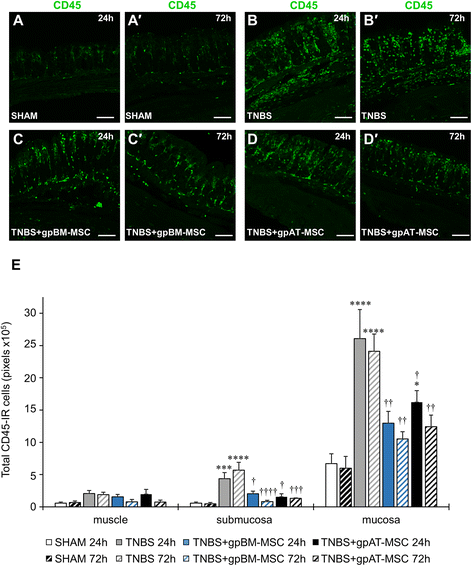
Methods: MSCs from guinea pig bone marrow and adipose tissue were isolated and characterised in vitro. In in vivo experiments, guinea pigs received either TNBS for the induction of colitis or sham treatment by enema. MSCs were administered at a dose of 1 × 10(6) cells via enema 3 h after the induction of colitis. Colon tissues were collected 24 and 72 h after TNBS administration to assess the level of inflammation and damage to the ENS. The secretion of transforming growth factor-β1 (TGF-β1) was analysed in MSC conditioned medium by flow cytometry.
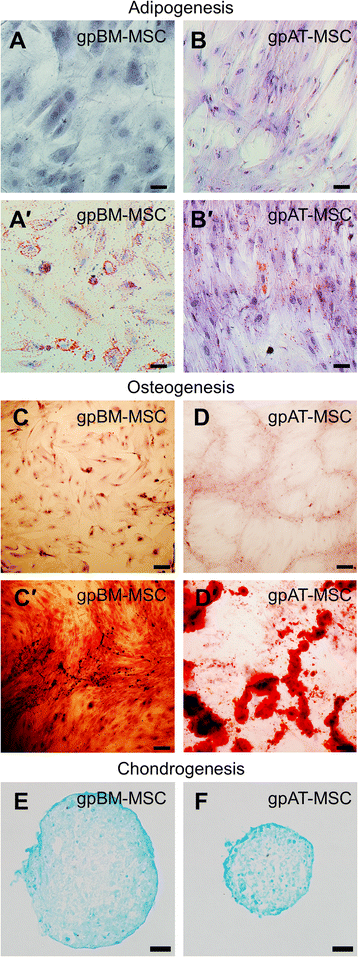
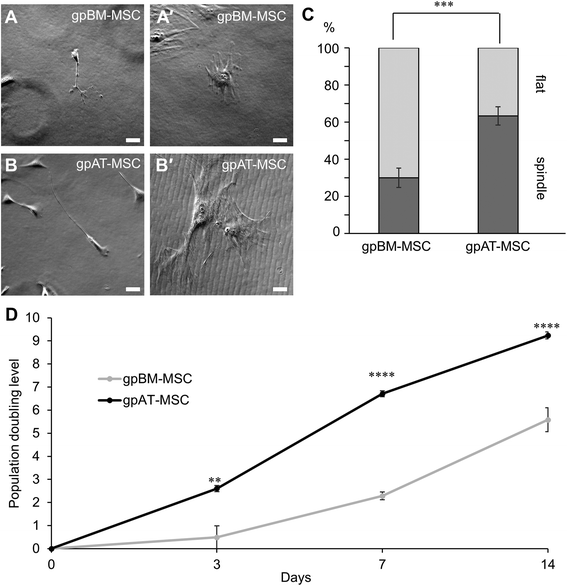
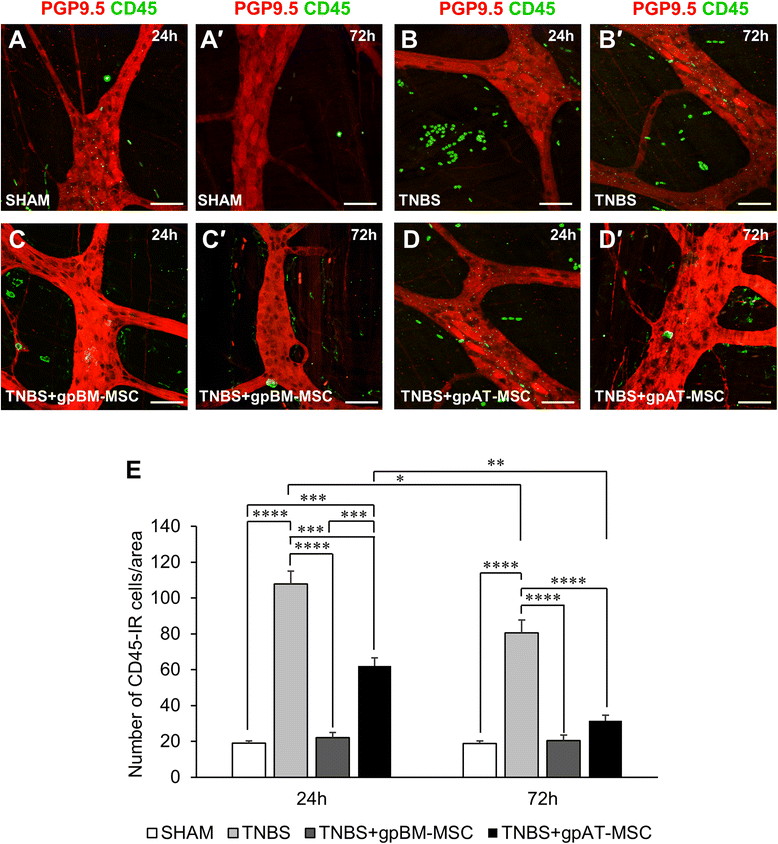
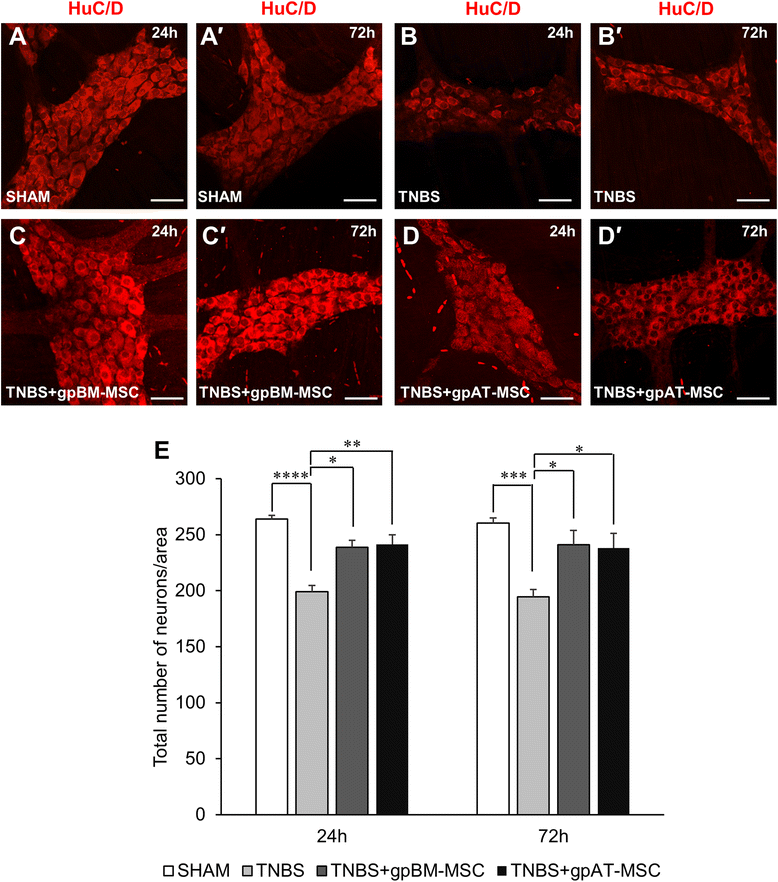
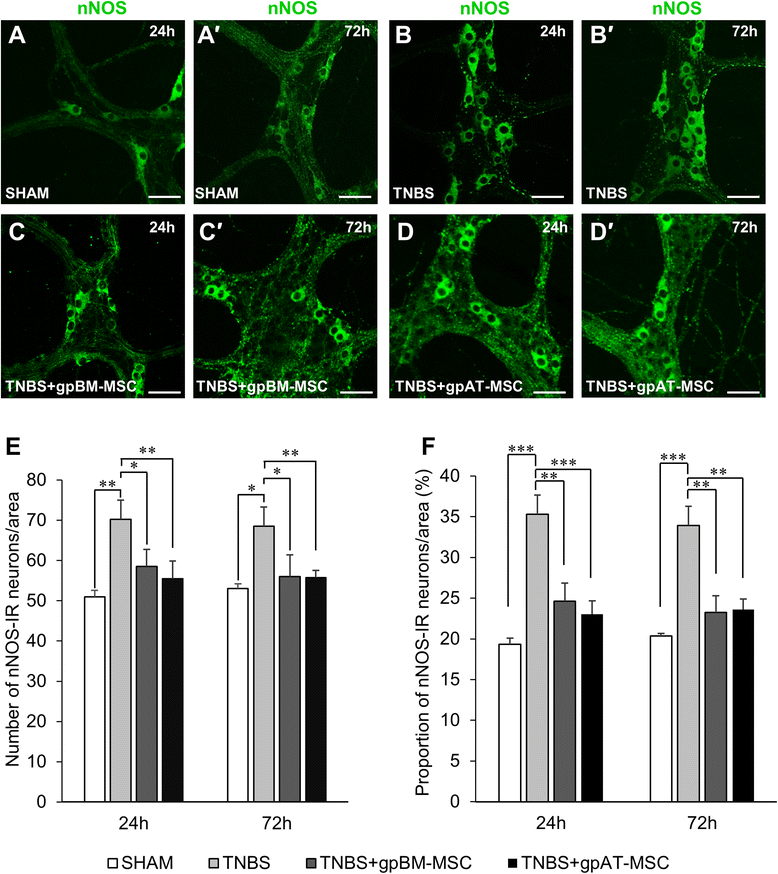
Results: Cells isolated from both sources were adherent to plastic, multipotent and expressed some human MSC surface markers. In vitro characterisation revealed distinct differences in growth kinetics, clonogenicity and cell morphology between MSC types. In an in vivo model of TNBS-induced colitis, guinea pig bone marrow MSCs were comparatively more efficacious than adipose tissue MSCs in attenuating weight loss, colonic tissue damage and leukocyte infiltration into the mucosa and myenteric plexus. MSCs from both sources were equally neuroprotective in the amelioration of enteric neuronal loss and changes to the neurochemical coding of neuronal subpopulations. MSCs from both sources secreted TGF-β1 which exerted neuroprotective effects in vitro.
Conclusions: This study is the first evaluating the functional capacity of guinea pig bone marrow and adipose tissue-derived MSCs and providing evidence of their neuroprotective value in an animal model of colitis. In vitro characteristics of MSCs cannot be extrapolated to their therapeutic efficacy. TGF-β1 released by both types of MSCs might have contributed to the attenuation of enteric neuropathy associated with colitis.
Figures



References
-
- Vester-Andersen MK, Prosberg MV, Jess T, Andersson M, Bengtsson BG, Blixt T, et al. Disease course and surgery rates in inflammatory bowel disease: a population-based, 7-year follow-up study in the era of immunomodulating therapy. Am J Gastroenterol. 2014;109:705–14. doi: 10.1038/ajg.2014.45. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

