Missing data in randomized controlled trials testing palliative interventions pose a significant risk of bias and loss of power: a systematic review and meta-analyses
- PMID: 26718729
- PMCID: PMC4910872
- DOI: 10.1016/j.jclinepi.2015.12.003
Missing data in randomized controlled trials testing palliative interventions pose a significant risk of bias and loss of power: a systematic review and meta-analyses
Abstract
Objectives: To assess the risk posed by missing data (MD) to the power and validity of trials evaluating palliative interventions.
Study design and setting: A systematic review of MD in published randomized controlled trials (RCTs) of palliative interventions in participants with life-limiting illnesses was conducted, and random-effects meta-analyses and metaregression were performed. CENTRAL, MEDLINE, and EMBASE (2009-2014) were searched with no language restrictions.
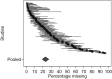
Results: One hundred and eight RCTs representing 15,560 patients were included. The weighted estimate for MD at the primary endpoint was 23.1% (95% confidence interval [CI] 19.3, 27.4). Larger MD proportions were associated with increasing numbers of questions/tests requested (odds ratio [OR], 1.19; 95% CI 1.05, 1.35) and with longer study duration (OR, 1.09; 95% CI 1.02, 1.17). Meta-analysis found evidence of differential rates of MD between trial arms, which varied in direction (OR, 1.04; 95% CI 0.90, 1.20; I(2) 35.9, P = 0.001). Despite randomization, MD in the intervention arms (vs. control) were more likely to be attributed to disease progression unrelated to the intervention (OR, 1.31; 95% CI 1.02, 1.69). This was not the case for MD due to death (OR, 0.92; 95% CI 0.78, 1.08).
Conclusion: The overall proportion and differential rates and reasons for MD reduce the power and potentially introduce bias to palliative care trials.
Keywords: Differential mortality; Meta-analysis; Missing data; Palliative care; Randomized controlled trials; Systematic review.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Carpenter J, Bartlett J, Kenward M. http://www.missingdata.org.uk Online: London School of Hygiene and Tropical Medicine. Available at: http://missingdata.lshtm.ac.uk. Accessed December 2, 2014.
-
- Raghunathan T.E. What do we do with missing data? Some options for analysis of incomplete data. Annu Rev Public Health. 2004;25:99–117. - PubMed
-
- Panel on Handling Missing Data in Clinical Trials . National Research Council; 2010. The prevention and treatment of missing data in clinical trials. Available at: http://www.cytel.com/hs-fs/hub/1670/file-2411099288-pdf/Pdf/MissingDataN.... Accessed January 8, 2016.
-
- Preston N.J., Fayers P., Walters S.J., Pilling M., Grande G.E., Short V. Recommendations for managing missing data, attrition and response shift in palliative and end-of-life care research: part of the MORECare research method guidance on statistical issues. Palliat Med. 2013;27:899–907. - PubMed
-
- Currow D.C., Plummer J.L., Kutner J.S., Samsa G.P., Abernethy A.P. Analyzing phase III studies in hospice/palliative care. A solution that sits between intention-to-treat and per protocol analyses: the palliative-modified ITT analysis. J Pain Symptom Manage. 2012;44:595–603. English. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical