The Karnataka Anemia Project 2--design and evaluation of a community-based parental intervention to improve childhood anemia cure rates: study protocol for a cluster randomized controlled trial
- PMID: 26718897
- PMCID: PMC4697328
- DOI: 10.1186/s13063-015-1135-x
The Karnataka Anemia Project 2--design and evaluation of a community-based parental intervention to improve childhood anemia cure rates: study protocol for a cluster randomized controlled trial
Abstract
Background: Childhood anemia is highly prevalent worldwide. Improving the hemoglobin level of preschool age children could yield substantial benefits in cognitive and psychosocial development and overall health. While evidence-based recommendations for reducing childhood anemia in high anemia prevalence countries are available, there is no experimental evidence of community centered education and counseling programs, as a route to improved acceptance of iron supplements, demonstrating beneficial effects on anemia outcomes. We report on the evaluation protocol of a complex educational intervention led by the community lay health worker (LHW) and delivered to mothers of 12-59-month-old anemic children living in and visiting village day care centers in a large district of southern India.
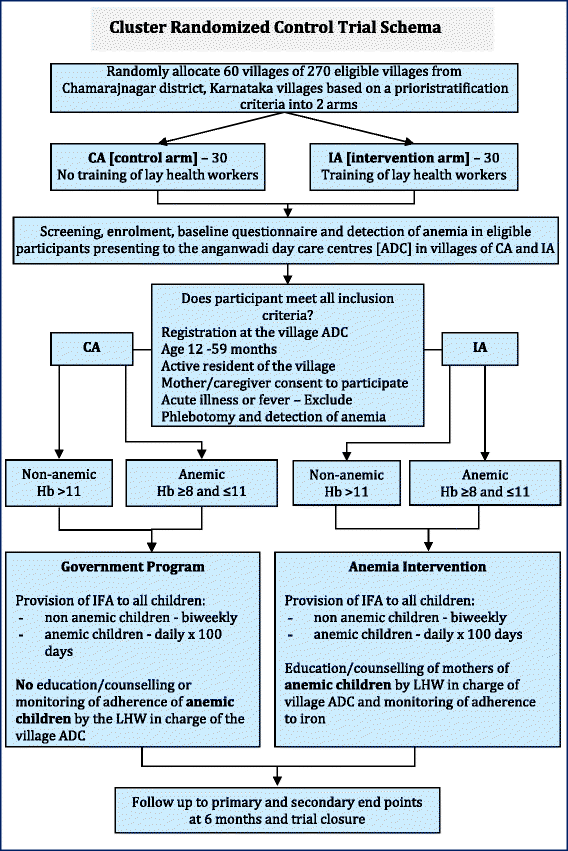
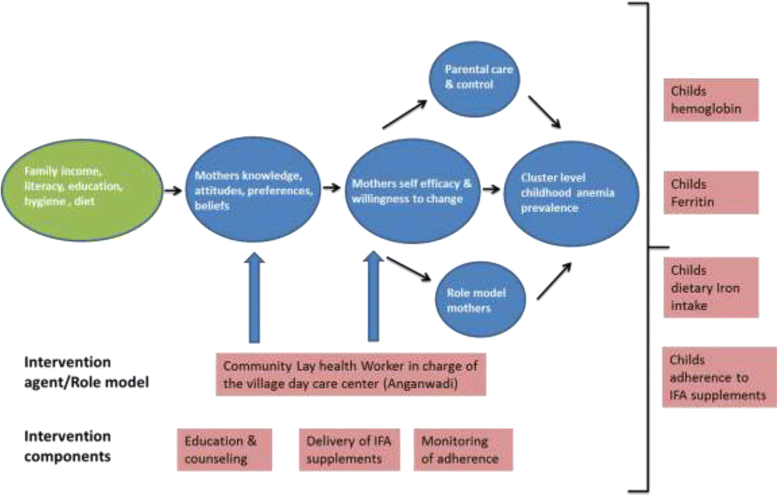
Methods and design: The study is designed as a cluster randomized controlled trial. The intervention is based on the social cognitive theory and aims to promote among mothers, anemia awareness, dietary modifications to increase iron intake in the child, and recognition of the need for enhanced adherence to supplemental iron in the anemic child. From 270 eligible villages in the study area, a sample of 60 villages will be randomized to intervention [n = 30] or to treatment as usual [n = 30] of the study. LHWs in the intervention arm will be trained to administer the following intervention components to mothers of anemic children: 1] monthly distribution of Iron and folic acid (IFA) supplements to mothers of anemic children, and 2] five monthly counseling sessions of mothers of anemic children covering: a] anemia awareness education b] IFA adherence counseling and assessment, c] dietary modification to improve iron intake, and d] hygiene and sanitation. LHWs in the control arm will distribute IFA to mothers of anemic children as in the intervention arm but will not provide monthly education and counseling support. The primary outcome is the difference between the two experimental groups in anemia cure rates of children found to be anemic at baseline. Secondary outcomes, assessed as differences between all participants in both experimental groups, are: change in mothers' knowledge regarding anemia; 24 hour dietary iron intake; net improvement in individual hemoglobin values; serum ferritin; and the difference in overall cluster level childhood anemia prevalence. All outcomes will be measured 6 months after the start of the intervention. Multilevel linear and logistic regression models will be used to analyze differences between intervention and control groups in outcome variables.
Discussion: This trial is designed to evaluate the effectiveness of an intervention intended to improve anemia cure rates in anemic children living in villages of Chamarajnagar, Karnataka a large district in south India. The extensive study of secondary endpoints will be used to identify possible weak points in the compliance to intervention delivery and uptake. This evaluation is one of the few large randomized trials evaluating the impact of an education and counseling intervention to reduce childhood anemia prevalence.
Trial registration: This trial was registered with ISRCTN.com (identifier: ISRCTN68413407) on 17 September 2013.
Figures
Similar articles
-
Lay health workers perceptions of an anemia control intervention in Karnataka, India: a qualitative study.BMC Public Health. 2017 Sep 18;17(1):720. doi: 10.1186/s12889-017-4758-x. BMC Public Health. 2017. PMID: 28923041 Free PMC article. Clinical Trial.
-
Effect of a maternal counselling intervention delivered by community health workers on child nutrition: secondary analysis of a cluster randomised controlled trial in India.BMC Public Health. 2021 Nov 5;21(1):2015. doi: 10.1186/s12889-021-11998-w. BMC Public Health. 2021. PMID: 34740351 Free PMC article. Clinical Trial.
-
Effect of a Community Health Worker-Delivered Parental Education and Counseling Intervention on Anemia Cure Rates in Rural Indian Children: A Pragmatic Cluster Randomized Clinical Trial.JAMA Pediatr. 2019 Sep 1;173(9):826-834. doi: 10.1001/jamapediatrics.2019.2087. JAMA Pediatr. 2019. PMID: 31329246 Free PMC article.
-
Interventions to improve disposal of child faeces for preventing diarrhoea and soil-transmitted helminth infection.Cochrane Database Syst Rev. 2019 Sep 24;9(9):CD011055. doi: 10.1002/14651858.CD011055.pub2. Cochrane Database Syst Rev. 2019. PMID: 31549742 Free PMC article.
-
Community-based maternal and newborn educational care packages for improving neonatal health and survival in low- and middle-income countries.Cochrane Database Syst Rev. 2019 Nov 5;2019(11):CD007647. doi: 10.1002/14651858.CD007647.pub2. Cochrane Database Syst Rev. 2019. PMID: 31686427 Free PMC article.
Cited by
-
Scaling-up high-impact micronutrient supplementation interventions to improve adolescents' nutrition and health in Burkina Faso and Tanzania: protocol for a cluster-randomised controlled trial.BMJ Open. 2023 Feb 15;13(2):e063686. doi: 10.1136/bmjopen-2022-063686. BMJ Open. 2023. PMID: 36792333 Free PMC article.
-
Lay health workers perceptions of an anemia control intervention in Karnataka, India: a qualitative study.BMC Public Health. 2017 Sep 18;17(1):720. doi: 10.1186/s12889-017-4758-x. BMC Public Health. 2017. PMID: 28923041 Free PMC article. Clinical Trial.
-
The Importance of Iron Status for Young Children in Low- and Middle-Income Countries: A Narrative Review.Pharmaceuticals (Basel). 2019 Apr 16;12(2):59. doi: 10.3390/ph12020059. Pharmaceuticals (Basel). 2019. PMID: 30995720 Free PMC article. Review.
-
School Teachers' Perspectives on National Iron Plus Initiative Implementation: A Qualitative Study.Indian Pediatr. 2025 Jun;62(6):421-427. doi: 10.1007/s13312-025-00039-z. Epub 2025 Apr 2. Indian Pediatr. 2025. PMID: 40172590
-
Effect of a maternal counselling intervention delivered by community health workers on child nutrition: secondary analysis of a cluster randomised controlled trial in India.BMC Public Health. 2021 Nov 5;21(1):2015. doi: 10.1186/s12889-021-11998-w. BMC Public Health. 2021. PMID: 34740351 Free PMC article. Clinical Trial.
References
-
- de Benoist B, McLean E, Egli I, Cogswell M. Worldwide prevalence of anaemia 1993–2005. WHO global database on anaemia; 2008. http://apps.who.int/iris/bitstream/10665/43894/1/9789241596657_eng.pdf. - PubMed
-
- Stevens GA, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F, Peña-Rosas JP, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Global Health. 2013;1:e16–25. doi: 10.1016/S2214-109X(13)70001-9. - DOI - PMC - PubMed
-
- Horton SRJ. The economics of iron deficiency. Food Policy. 2003;28:51–75. doi: 10.1016/S0306-9192(02)00070-2. - DOI
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical