Absence of AMPKα2 accelerates cellular senescence via p16 induction in mouse embryonic fibroblasts
- PMID: 26718972
- PMCID: PMC4720555
- DOI: 10.1016/j.biocel.2015.12.010
Absence of AMPKα2 accelerates cellular senescence via p16 induction in mouse embryonic fibroblasts
Abstract
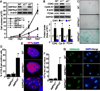
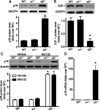
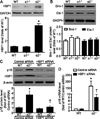
Emerging evidence suggests that activation of adenosine monophosphate-activated protein kinase (AMPK), an energy gauge and redox sensor, delays aging process. However, the molecular mechanisms by which AMPKα isoform regulates cellular senescence remain largely unknown. The aim of this study was to determine if AMPKα deletion contributes to the accelerated cell senescence by inducing p16(INK4A) (p16) expression thereby arresting cell cycle. The markers of cellular senescence, cell cycle proteins, and reactive oxygen species (ROS) were monitored in cultured mouse embryonic fibroblasts (MEFs) isolated from wild type (WT, C57BL/6J), AMPKα1, or AMPKα2 homozygous deficient (AMPKα1(-/-), AMPKα2(-/-)) mice by Western blot and cellular immunofluorescence staining, as well as immunohistochemistry (IHC) in skin tissue of young and aged mice. Deletion of AMPKα2, the minor isoform of AMPKα, but not AMPKα1 in high-passaged MEFs led to spontaneous cell senescence demonstrated by accumulation of senescence-associated-β-galactosidase (SA-β-gal) staining and foci formation of heterochromatin protein 1 homolog gamma (HP1γ). It was shown here that AMPKα2 deletion upregulates cyclin-dependent kinase (CDK) inhibitor, p16, which arrests cell cycle. Furthermore, AMPKα2 null cells exhibited elevated ROS production. Interestingly, knockdown of HMG box-containing protein 1 (HBP1) partially blocked the cellular senescence of AMPKα2-deleted MEFs via the reduction of p16. Finally, dermal cells senescence, including fibroblasts senescence evidenced by the staining of p16, HBP1, and Ki-67, in the skin of aged AMPKα2(-/-) mice was enhanced when compared with that in wild type mice. Taken together, our results suggest that AMPKα2 isoform plays a fundamental role in anti-oxidant stress and anti-senescence.
Keywords: AMPKα2; Cellular senescence; HBP1; Reactive oxygen species; p16.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures




Similar articles
-
AMPKα1 deficiency promotes cellular proliferation and DNA damage via p21 reduction in mouse embryonic fibroblasts.Biochim Biophys Acta. 2015 Jan;1853(1):65-73. doi: 10.1016/j.bbamcr.2014.10.002. Epub 2014 Oct 13. Biochim Biophys Acta. 2015. PMID: 25307521 Free PMC article.
-
Loss of AMPKalpha1 Triggers Centrosome Amplification via PLK4 Upregulation in Mouse Embryonic Fibroblasts.Int J Mol Sci. 2020 Apr 16;21(8):2772. doi: 10.3390/ijms21082772. Int J Mol Sci. 2020. PMID: 32316320 Free PMC article.
-
Transcriptional factor HBP1 targets P16(INK4A), upregulating its expression and consequently is involved in Ras-induced premature senescence.Oncogene. 2010 Sep 9;29(36):5083-94. doi: 10.1038/onc.2010.252. Epub 2010 Jun 28. Oncogene. 2010. PMID: 20581871
-
Jun dimerization protein 2 in oxygen restriction; control of senescence.Curr Pharm Des. 2011;17(22):2278-89. doi: 10.2174/138161211797052394. Curr Pharm Des. 2011. PMID: 21736542 Review.
-
Transcriptional Regulation of the p16 Tumor Suppressor Gene.Anticancer Res. 2015 Aug;35(8):4397-401. Anticancer Res. 2015. PMID: 26168478 Review.
Cited by
-
AMPK-mediated senolytic and senostatic activity of quercetin surface functionalized Fe3O4 nanoparticles during oxidant-induced senescence in human fibroblasts.Redox Biol. 2020 Jan;28:101337. doi: 10.1016/j.redox.2019.101337. Epub 2019 Oct 4. Redox Biol. 2020. PMID: 31622846 Free PMC article.
-
Activation of AMP-activated protein kinase by metformin ablates angiotensin II-induced endoplasmic reticulum stress and hypertension in mice in vivo.Br J Pharmacol. 2017 Jul;174(13):2140-2151. doi: 10.1111/bph.13833. Epub 2017 May 31. Br J Pharmacol. 2017. PMID: 28436023 Free PMC article.
-
AMPKα1 deletion in fibroblasts promotes tumorigenesis in athymic nude mice by p52-mediated elevation of erythropoietin and CDK2.Oncotarget. 2016 Aug 16;7(33):53654-53667. doi: 10.18632/oncotarget.10687. Oncotarget. 2016. PMID: 27449088 Free PMC article.
-
Senescence-associated-β-galactosidase staining following traumatic brain injury in the mouse cerebrum.PLoS One. 2019 Mar 11;14(3):e0213673. doi: 10.1371/journal.pone.0213673. eCollection 2019. PLoS One. 2019. PMID: 30856215 Free PMC article.
-
Protective Effect of Hyperbaric Oxygen on Cognitive Impairment Induced by D-Galactose in Mice.Neurochem Res. 2016 Nov;41(11):3032-3041. doi: 10.1007/s11064-016-2022-x. Epub 2016 Aug 3. Neurochem Res. 2016. PMID: 27485714
References
-
- Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AH, Schlegelberger B, Stein H, Dorken B, Jenuwein T, Schmitt CA. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–665. - PubMed
-
- Branchet MC, Boisnic S, Frances C, Robert AM. Skin thickness changes in normal aging skin. Gerontology. 1990;36:28–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL110488/HL/NHLBI NIH HHS/United States
- R01 HL089920/HL/NHLBI NIH HHS/United States
- R01 HL128014/HL/NHLBI NIH HHS/United States
- R01 HL074399/HL/NHLBI NIH HHS/United States
- HL089920/HL/NHLBI NIH HHS/United States
- HL080499/HL/NHLBI NIH HHS/United States
- R01 AG047776/AG/NIA NIH HHS/United States
- HL079584/HL/NHLBI NIH HHS/United States
- HL105157/HL/NHLBI NIH HHS/United States
- AG047776/AG/NIA NIH HHS/United States
- R01 HL110488/HL/NHLBI NIH HHS/United States
- R01 HL105157/HL/NHLBI NIH HHS/United States
- R01 HL079584/HL/NHLBI NIH HHS/United States
- R01 HL080499/HL/NHLBI NIH HHS/United States
- R01 HL096032/HL/NHLBI NIH HHS/United States
- HL096032/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

