Synaptic Amyloid-β Oligomers Precede p-Tau and Differentiate High Pathology Control Cases
- PMID: 26718979
- PMCID: PMC4715217
- DOI: 10.1016/j.ajpath.2015.09.018
Synaptic Amyloid-β Oligomers Precede p-Tau and Differentiate High Pathology Control Cases
Abstract
Amyloid-β (Aβ) and hyperphosphorylated tau (p-tau) aggregates form the two discrete pathologies of Alzheimer disease (AD), and oligomeric assemblies of each protein are localized to synapses. To determine the sequence by which pathology appears in synapses, Aβ and p-tau were quantified across AD disease stages in parietal cortex. Nondemented cases with high levels of AD-related pathology were included to determine factors that confer protection from clinical symptoms. Flow cytometric analysis of synaptosome preparations was used to quantify Aβ and p-tau in large populations of individual synaptic terminals. Soluble Aβ oligomers were assayed by a single antibody sandwich enzyme-linked immunosorbent assay. Total in situ Aβ was elevated in patients with early- and late-stage AD dementia, but not in high pathology nondemented controls compared with age-matched normal controls. However, soluble Aβ oligomers were highest in early AD synapses, and this assay distinguished early AD cases from high pathology controls. Overall, synapse-associated p-tau did not increase until late-stage disease in human and transgenic rat cortex, and p-tau was elevated in individual Aβ-positive synaptosomes in early AD. These results suggest that soluble oligomers in surviving neocortical synaptic terminals are associated with dementia onset and suggest an amyloid cascade hypothesis in which oligomeric Aβ drives phosphorylated tau accumulation and synaptic spread. These results indicate that antiamyloid therapies will be less effective once p-tau pathology is developed.
Copyright © 2016 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures




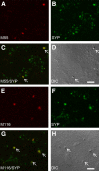

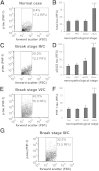


References
-
- Hefti F., Goure W.F., Jerecic J., Iverson K.S., Walicke P.A., Krafft G.A. The case for soluble Abeta oligomers as a drug target in Alzheimer's disease. Trends Pharmacol Sci. 2013;34:261–266. - PubMed
-
- Arriagada P.V., Growdon J.H., Hedley-Whyte E.T., Hyman B.T. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–639. - PubMed
-
- Bierer L.M., Hof P.R., Purohit D.P., Carlin L., Schmeidler J., Davis K.L., Perl D.P. Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer's disease. Arch Neurol. 1995;52:81–88. - PubMed
-
- Giannakopoulos P., Herrmann F.R., Bussiere T., Bouras C., Kovari E., Perl D.P., Morrison J.H., Gold G., Hof P.R. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer's disease. Neurology. 2003;60:1495–1500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG018879/AG/NIA NIH HHS/United States
- P50 AG16970/AG/NIA NIH HHS/United States
- AG34628/AG/NIA NIH HHS/United States
- R56 AG027465/AG/NIA NIH HHS/United States
- R01 AG041295/AG/NIA NIH HHS/United States
- UL1 TR000124/TR/NCATS NIH HHS/United States
- AG18879/AG/NIA NIH HHS/United States
- R21 AG051946/AG/NIA NIH HHS/United States
- CA16042/CA/NCI NIH HHS/United States
- R01 AG027465/AG/NIA NIH HHS/United States
- K08 AG034628/AG/NIA NIH HHS/United States
- R01 NS038328/NS/NINDS NIH HHS/United States
- P50 AG016573/AG/NIA NIH HHS/United States
- P50 AG005142/AG/NIA NIH HHS/United States
- NS038328/NS/NINDS NIH HHS/United States
- P30 CA016042/CA/NCI NIH HHS/United States
- P50 AG05142/AG/NIA NIH HHS/United States
- AI 28697/AI/NIAID NIH HHS/United States
- R01AG21055/AG/NIA NIH HHS/United States
- UL1TR000124/TR/NCATS NIH HHS/United States
- AG041295/AG/NIA NIH HHS/United States
- AG27465/AG/NIA NIH HHS/United States
- P50 AG16573/AG/NIA NIH HHS/United States
- P30 AI028697/AI/NIAID NIH HHS/United States
- R01 AG021055/AG/NIA NIH HHS/United States
- P01 AG000538/AG/NIA NIH HHS/United States
- P30 AG028748/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

