Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial
- PMID: 26719232
- PMCID: PMC4800035
- DOI: 10.1016/S0140-6736(15)01037-5
Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial
Abstract
Background: Long-term hormone therapy has been the standard of care for advanced prostate cancer since the 1940s. STAMPEDE is a randomised controlled trial using a multiarm, multistage platform design. It recruits men with high-risk, locally advanced, metastatic or recurrent prostate cancer who are starting first-line long-term hormone therapy. We report primary survival results for three research comparisons testing the addition of zoledronic acid, docetaxel, or their combination to standard of care versus standard of care alone.
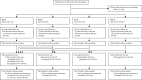
Methods: Standard of care was hormone therapy for at least 2 years; radiotherapy was encouraged for men with N0M0 disease to November, 2011, then mandated; radiotherapy was optional for men with node-positive non-metastatic (N+M0) disease. Stratified randomisation (via minimisation) allocated men 2:1:1:1 to standard of care only (SOC-only; control), standard of care plus zoledronic acid (SOC + ZA), standard of care plus docetaxel (SOC + Doc), or standard of care with both zoledronic acid and docetaxel (SOC + ZA + Doc). Zoledronic acid (4 mg) was given for six 3-weekly cycles, then 4-weekly until 2 years, and docetaxel (75 mg/m(2)) for six 3-weekly cycles with prednisolone 10 mg daily. There was no blinding to treatment allocation. The primary outcome measure was overall survival. Pairwise comparisons of research versus control had 90% power at 2·5% one-sided α for hazard ratio (HR) 0·75, requiring roughly 400 control arm deaths. Statistical analyses were undertaken with standard log-rank-type methods for time-to-event data, with hazard ratios (HRs) and 95% CIs derived from adjusted Cox models. This trial is registered at ClinicalTrials.gov (NCT00268476) and ControlledTrials.com (ISRCTN78818544).
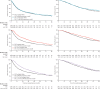
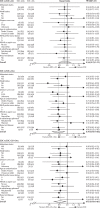
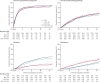
Findings: 2962 men were randomly assigned to four groups between Oct 5, 2005, and March 31, 2013. Median age was 65 years (IQR 60-71). 1817 (61%) men had M+ disease, 448 (15%) had N+/X M0, and 697 (24%) had N0M0. 165 (6%) men were previously treated with local therapy, and median prostate-specific antigen was 65 ng/mL (IQR 23-184). Median follow-up was 43 months (IQR 30-60). There were 415 deaths in the control group (347 [84%] prostate cancer). Median overall survival was 71 months (IQR 32 to not reached) for SOC-only, not reached (32 to not reached) for SOC + ZA (HR 0·94, 95% CI 0·79-1·11; p=0·450), 81 months (41 to not reached) for SOC + Doc (0·78, 0·66-0·93; p=0·006), and 76 months (39 to not reached) for SOC + ZA + Doc (0·82, 0·69-0·97; p=0·022). There was no evidence of heterogeneity in treatment effect (for any of the treatments) across prespecified subsets. Grade 3-5 adverse events were reported for 399 (32%) patients receiving SOC, 197 (32%) receiving SOC + ZA, 288 (52%) receiving SOC + Doc, and 269 (52%) receiving SOC + ZA + Doc.
Interpretation: Zoledronic acid showed no evidence of survival improvement and should not be part of standard of care for this population. Docetaxel chemotherapy, given at the time of long-term hormone therapy initiation, showed evidence of improved survival accompanied by an increase in adverse events. Docetaxel treatment should become part of standard of care for adequately fit men commencing long-term hormone therapy.
Funding: Cancer Research UK, Medical Research Council, Novartis, Sanofi-Aventis, Pfizer, Janssen, Astellas, NIHR Clinical Research Network, Swiss Group for Clinical Cancer Research.
Copyright © 2016 James et al. Open Access article distributed under the terms of CC BY. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
Prostate cancer: Changing standard of care in hormone-sensitive disease.Nat Rev Urol. 2016 Feb;13(2):61. doi: 10.1038/nrurol.2016.4. Epub 2016 Jan 20. Nat Rev Urol. 2016. PMID: 26787390 No abstract available.
-
Defining new standards of care for men with prostate cancer.Lancet. 2016 Mar 19;387(10024):1135-7. doi: 10.1016/S0140-6736(15)01235-0. Lancet. 2016. PMID: 27025318 No abstract available.
-
High Risk of Neutropenia for Hormone-naive Prostate Cancer Patients Receiving STAMPEDE-style Upfront Docetaxel Chemotherapy in Usual Clinical Practice.Clin Oncol (R Coll Radiol). 2016 Sep;28(9):611. doi: 10.1016/j.clon.2016.03.006. Epub 2016 May 4. Clin Oncol (R Coll Radiol). 2016. PMID: 27131755 No abstract available.
-
Ten Years on the Juggernaut Keeps on Rolling: Comments on the STAMPEDE Trial from the Front Line.Clin Oncol (R Coll Radiol). 2016 Sep;28(9):547-9. doi: 10.1016/j.clon.2016.04.047. Epub 2016 May 25. Clin Oncol (R Coll Radiol). 2016. PMID: 27236422 No abstract available.
-
Re: Addition of Docetaxel, Zoledronic Acid, or Both to First-line Long-term Hormone Therapy in Prostate Cancer (STAMPEDE): Survival Results from an Adaptive, Multiarm, Multistage, Platform Randomised Controlled Trial.Eur Urol. 2016 Jun;69(6):1155-6. doi: 10.1016/j.eururo.2016.02.022. Eur Urol. 2016. PMID: 27302137 No abstract available.
-
STAMPEDE trial and patients with non-metastatic prostate cancer.Lancet. 2016 Jul 16;388(10041):234-5. doi: 10.1016/S0140-6736(16)31038-8. Lancet. 2016. PMID: 27479564 No abstract available.
-
STAMPEDE trial and patients with non-metastatic prostate cancer - Authors' reply.Lancet. 2016 Jul 16;388(10041):235-6. doi: 10.1016/S0140-6736(16)31041-8. Lancet. 2016. PMID: 27479566 No abstract available.
-
Re: Addition of Docetaxel, Zoledronic Acid, or Both to First-Line Long-Term Hormone Therapy in Prostate Cancer (STAMPEDE): Survival Results from an Adaptive, Multiarm, Multistage, Platform Randomised Controlled Trial.J Urol. 2016 Oct;196(4):1124-5. doi: 10.1016/j.juro.2016.07.067. Epub 2016 Jul 18. J Urol. 2016. PMID: 27628795 No abstract available.
-
Zoledronic Acid in First-Line Treatment of Prostate Cancer.Int J Radiat Oncol Biol Phys. 2017 Jan 1;97(1):6-8. doi: 10.1016/j.ijrobp.2016.06.2453. Int J Radiat Oncol Biol Phys. 2017. PMID: 27979458 No abstract available.
-
Chemotherapy should not yet be considered in patients with hormono-sensitive metastatic prostate cáncer.Actas Urol Esp. 2017 Jul-Aug;41(6):347-351. doi: 10.1016/j.acuro.2016.11.008. Epub 2017 Feb 14. Actas Urol Esp. 2017. PMID: 28214037 English, Spanish. No abstract available.
-
Treatment of metastatic prostate cancer after STAMPEDE.Transl Androl Urol. 2017 Apr;6(2):315-316. doi: 10.21037/tau.2017.02.01. Transl Androl Urol. 2017. PMID: 28540244 Free PMC article. No abstract available.
-
Prostate cancer: A new standard-of-care for advanced-stage disease.Nat Rev Clin Oncol. 2017 Oct;14(10):592-593. doi: 10.1038/nrclinonc.2017.120. Epub 2017 Aug 8. Nat Rev Clin Oncol. 2017. PMID: 28786417 No abstract available.
References
-
- Petrylak DP, Tangen CM, Hussain MH. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. - PubMed
-
- Tannock IF, de Wit R, Berry WR. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. - PubMed
-
- Scher HI, Fizazi K, Saad F. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

