Alpha-Synuclein Expression Restricts RNA Viral Infections in the Brain
- PMID: 26719256
- PMCID: PMC4810656
- DOI: 10.1128/JVI.02949-15
Alpha-Synuclein Expression Restricts RNA Viral Infections in the Brain
Abstract
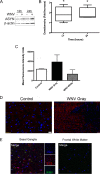
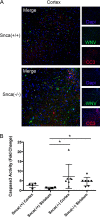
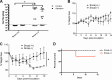
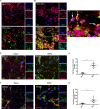
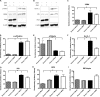
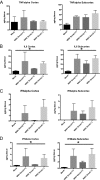
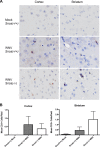
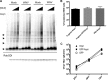
We have discovered that native, neuronal expression of alpha-synuclein (Asyn) inhibits viral infection, injury, and disease in the central nervous system (CNS). Enveloped RNA viruses, such as West Nile virus (WNV), invade the CNS and cause encephalitis, yet little is known about the innate neuron-specific inhibitors of viral infections in the CNS. Following WNV infection of primary neurons, we found that Asyn protein expression is increased. The infectious titer of WNV and Venezuelan equine encephalitis virus (VEEV) TC83 in the brains of Asyn-knockout mice exhibited a mean increase of 10(4.5) infectious viral particles compared to the titers in wild-type and heterozygote littermates. Asyn-knockout mice also exhibited significantly increased virus-induced mortality compared to Asyn heterozygote or homozygote control mice. Virus-induced Asyn localized to perinuclear, neuronal regions expressing viral envelope protein and the endoplasmic reticulum (ER)-associated trafficking protein Rab1. In Asyn-knockout primary neuronal cultures, the levels of expression of ER signaling pathways, known to support WNV replication, were significantly elevated before and during viral infection compared to those in Asyn-expressing primary neuronal cultures. We propose a model in which virus-induced Asyn localizes to ER-derived membranes, modulates virus-induced ER stress signaling, and inhibits viral replication, growth, and injury in the CNS. These data provide a novel and important functional role for the expression of native alpha-synuclein, a protein that is closely associated with the development of Parkinson's disease.
Importance: Neuroinvasive viruses such as West Nile virus are able to infect neurons and cause severe disease, such as encephalitis, or infection of brain tissue. Following viral infection in the central nervous system, only select neurons are infected, implying that neurons exhibit innate resistance to viral infections. We discovered that native neuronal expression of alpha-synuclein inhibited viral infection in the central nervous system. When the gene for alpha-synuclein was deleted, mice exhibited significantly decreased survival, markedly increased viral growth in the brain, and evidence of increased neuron injury. Virus-induced alpha-synuclein localized to intracellular neuron membranes, and in the absence of alpha-synuclein expression, specific endoplasmic reticulum stress signaling events were significantly increased. We describe a new neuron-specific inhibitor of viral infections in the central nervous system. Given the importance of alpha-synuclein as a cause of Parkinson's disease, these data also ascribe a novel functional role for the native expression of alpha-synuclein in the CNS.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Carrera JP, Forrester N, Wang E, Vittor AY, Haddow AD, Lopez-Verges S, Abadia I, Castano E, Sosa N, Baez C, Estripeaut D, Diaz Y, Beltran D, Cisneros J, Cedeno HG, Travassos da Rosa AP, Hernandez H, Martinez-Torres AO, Tesh RB, Weaver SC. 2013. Eastern equine encephalitis in Latin America. N Engl J Med 369:732–744. doi: 10.1056/NEJMoa1212628. - DOI - PMC - PubMed
-
- Armah HB, Wang G, Omalu BI, Tesh RB, Gyure KA, Chute DJ, Smith RD, Dulai P, Vinters HV, Kleinschmidt-DeMasters BK, Wiley CA. 2007. Systemic distribution of West Nile virus infection: postmortem immunohistochemical study of six cases. Brain Pathol 17:354–362. doi: 10.1111/j.1750-3639.2007.00080.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

