Alveolar Macrophages Are a Prominent but Nonessential Target for Murine Cytomegalovirus Infecting the Lungs
- PMID: 26719275
- PMCID: PMC4810665
- DOI: 10.1128/JVI.02856-15
Alveolar Macrophages Are a Prominent but Nonessential Target for Murine Cytomegalovirus Infecting the Lungs
Abstract
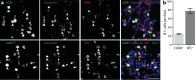
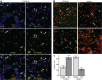
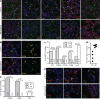
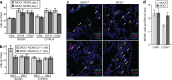
Cytomegaloviruses (CMVs) infect the lungs and cause pathological damage there in immunocompromised hosts. How lung infection starts is unknown. Inhaled murine CMV (MCMV) directly infected alveolar macrophages (AMs) and type 2 alveolar epithelial cells (AEC2s) but not type 1 alveolar epithelial cells (AEC1s). In contrast, herpes simplex virus 1 infected AEC1s and murid herpesvirus 4 (MuHV-4) infected AEC1s via AMs. MCMV-infected AMs prominently expressed viral reporter genes from a human CMV IE1 promoter; but most IE1-positive cells were AEC2s, and CD11c-cre mice, which express cre in AMs, switched the fluorochrome expression of <5% of floxed MCMV in the lungs. In contrast, CD11C-cre mice exhibited fluorochrome switching in >90% of floxed MuHV-4 in the lungs and 50% of floxed MCMV in the blood. AM depletion increased MCMV titers in the lung during the acute phase of infection. Thus, the influence of AMs was more restrictive than permissive. Circulating monocytes entered infected lungs in large numbers and became infected, but not directly; infection occurred mainly via AEC2s. Mice infected with an MCMV mutant lacking its m131/m129 chemokine homolog, which promotes macrophage infection, showed levels of lung infection equivalent to those of wild-type MCMV-infected mice. The level of lung infiltration by Gr-1-positive cells infected with the MCMV m131/m129-null mutant was modestly different from that for wild-type MCMV-infected lungs. These results are consistent with myeloid cells mainly disseminating MCMV from the lungs, whereas AEC2s provide local amplification.
Importance: Cytomegaloviruses (CMVs) chronically and systemically infect most mammals. Human CMV infection is usually asymptomatic but causes lung disease in people with poor immune function. As human infection is hard to analyze, studies with related animal viruses provide important insights. We show that murine CMV has two targets in the lungs: macrophages and surfactant-secreting epithelial cells. Acute virus replication occurred largely in epithelial cells. Macrophages had an important defensive role, as their removal increased the level of infection. These results establish the dual nature of lung infection, with local virus replication occurring in epithelial cells and spread occurring via quiescently infected macrophages. Distinct therapies may be needed to target these contrasting events.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures




Similar articles
-
Murine Cytomegalovirus Glycoprotein O Promotes Epithelial Cell Infection In Vivo.J Virol. 2019 Jan 17;93(3):e01378-18. doi: 10.1128/JVI.01378-18. Print 2019 Feb 1. J Virol. 2019. PMID: 30404805 Free PMC article.
-
Murine Cytomegalovirus Exploits Olfaction To Enter New Hosts.mBio. 2016 Apr 26;7(2):e00251-16. doi: 10.1128/mBio.00251-16. mBio. 2016. PMID: 27118588 Free PMC article.
-
Lymph Node Macrophages Restrict Murine Cytomegalovirus Dissemination.J Virol. 2015 Jul;89(14):7147-58. doi: 10.1128/JVI.00480-15. Epub 2015 Apr 29. J Virol. 2015. PMID: 25926638 Free PMC article.
-
[Cytomegalovirus infection and its possible treatment with herbal medicines].Nihon Rinsho. 1998 Jan;56(1):156-60. Nihon Rinsho. 1998. PMID: 9465682 Review. Japanese.
-
Mast cells: innate attractors recruiting protective CD8 T cells to sites of cytomegalovirus infection.Med Microbiol Immunol. 2015 Jun;204(3):327-34. doi: 10.1007/s00430-015-0386-1. Epub 2015 Feb 4. Med Microbiol Immunol. 2015. PMID: 25648117 Review.
Cited by
-
Dissecting the cytomegalovirus CC chemokine: Chemokine activity and gHgLchemokine-dependent cell tropism are independent players in CMV infection.PLoS Pathog. 2023 Dec 8;19(12):e1011793. doi: 10.1371/journal.ppat.1011793. eCollection 2023 Dec. PLoS Pathog. 2023. PMID: 38064525 Free PMC article.
-
Case report: Mafb promoter activity may define the alveolar macrophage dichotomy.Front Immunol. 2022 Dec 12;13:1050494. doi: 10.3389/fimmu.2022.1050494. eCollection 2022. Front Immunol. 2022. PMID: 36578483 Free PMC article.
-
Murine Cytomegalovirus Spreads by Dendritic Cell Recirculation.mBio. 2017 Oct 3;8(5):e01264-17. doi: 10.1128/mBio.01264-17. mBio. 2017. PMID: 28974616 Free PMC article.
-
Mouse cytomegalovirus lacking sgg1 shows reduced import into the salivary glands.J Gen Virol. 2024 Aug;105(8):002013. doi: 10.1099/jgv.0.002013. J Gen Virol. 2024. PMID: 39093048 Free PMC article.
-
Cytomegalovirus (CMV) Pneumonitis: Cell Tropism, Inflammation, and Immunity.Int J Mol Sci. 2019 Aug 8;20(16):3865. doi: 10.3390/ijms20163865. Int J Mol Sci. 2019. PMID: 31398860 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

