Structural and Mechanistic Insights into the Regulation of the Fundamental Rho Regulator RhoGDIα by Lysine Acetylation
- PMID: 26719334
- PMCID: PMC4786691
- DOI: 10.1074/jbc.M115.707091
Structural and Mechanistic Insights into the Regulation of the Fundamental Rho Regulator RhoGDIα by Lysine Acetylation
Abstract
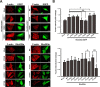
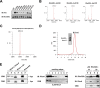
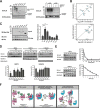
Rho proteins are small GTP/GDP-binding proteins primarily involved in cytoskeleton regulation. Their GTP/GDP cycle is often tightly connected to a membrane/cytosol cycle regulated by the Rho guanine nucleotide dissociation inhibitor α (RhoGDIα). RhoGDIα has been regarded as a housekeeping regulator essential to control homeostasis of Rho proteins. Recent proteomic screens showed that RhoGDIα is extensively lysine-acetylated. Here, we present the first comprehensive structural and mechanistic study to show how RhoGDIα function is regulated by lysine acetylation. We discover that lysine acetylation impairs Rho protein binding and increases guanine nucleotide exchange factor-catalyzed nucleotide exchange on RhoA, these two functions being prerequisites to constitute a bona fide GDI displacement factor. RhoGDIα acetylation interferes with Rho signaling, resulting in alteration of cellular filamentous actin. Finally, we discover that RhoGDIα is endogenously acetylated in mammalian cells, and we identify CBP, p300, and pCAF as RhoGDIα-acetyltransferases and Sirt2 and HDAC6 as specific deacetylases, showing the biological significance of this post-translational modification.
Keywords: Ras homolog gene family, member A (RhoA); Rho (Rho GTPase); acetylation; acetyltransferase; guanine-nucleotide-dissociation inhibitor alpha; histone deacetylase (HDAC); lysine acetylation; lysine acetyltransferase; post-translational modification (PTM); sirtuin.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Hall A. (1998) Rho GTPases and the actin cytoskeleton. Science 279, 509–514 - PubMed
-
- Gundersen G. G., Wen Y., Eng C. H., Schmoranzer J., Cabrera-Poch N., Morris E. J., Chen M., and Gomes E. R. (2005) Regulation of microtubules by Rho GTPases in migrating cells. Novartis Found. Symp. 269, 106–116; discussion 116–126, 223–230 - PubMed
-
- Jaffe A. B., and Hall A. (2005) Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21, 247–269 - PubMed
-
- Vetter I. R., and Wittinghofer A. (2001) The guanine nucleotide-binding switch in three dimensions. Science 294, 1299–1304 - PubMed
-
- Bos J. L., Rehmann H., and Wittinghofer A. (2007) GEFs and GAPs: critical elements in the control of small G proteins. Cell 129, 865–877 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

