Cyclable Condensation and Hierarchical Assembly of Metastable Reflectin Proteins, the Drivers of Tunable Biophotonics
- PMID: 26719342
- PMCID: PMC4759182
- DOI: 10.1074/jbc.M115.686014
Cyclable Condensation and Hierarchical Assembly of Metastable Reflectin Proteins, the Drivers of Tunable Biophotonics
Abstract
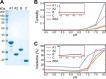
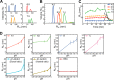
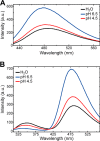
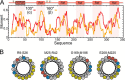
Reversible changes in the phosphorylation of reflectin proteins have been shown to drive the tunability of color and brightness of light reflected from specialized cells in the skin of squids and related cephalopods. We show here, using dynamic light scattering, electron microscopy, and fluorescence analyses, that reversible titration of the excess positive charges of the reflectins, comparable with that produced by phosphorylation, is sufficient to drive the reversible condensation and hierarchical assembly of these proteins. The results suggest a two-stage process in which charge neutralization first triggers condensation, resulting in the emergence of previously cryptic structures that subsequently mediate reversible, hierarchical assembly. The extent to which cyclability is seen in the in vitro formation and disassembly of complexes estimated to contain several thousand reflectin molecules suggests that intrinsic sequence- and structure-determined specificity governs the reversible condensation and assembly of the reflectins and that these processes are therefore sufficient to produce the reversible changes in refractive index, thickness, and spacing of the reflectin-containing subcellular Bragg lamellae to change the brightness and color of reflected light. This molecular mechanism points to the metastability of reflectins as the centrally important design principle governing biophotonic tunability in this system.
Keywords: biomaterials; biophotonics; intrinsically disordered protein; iridescence; protein aggregation; protein assembly; protein metastability; protein self-assembly; reflectins; tunable.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures






References
-
- Boal J. G., Shashar N., Grable M. M., Vaughan K. H., Loew E. R., and Hanlon R. T. (2004) Behavioral evidence for intraspecific signaling with achromatic and polarized light by cuttlefish (Mollusca: Cephalopoda). Behavior 141, 837–861
-
- Crookes W. J., Ding L.-L., Huang Q. L., Kimbell J. R., Horwitz J., and McFall-Ngai M. J. (2004) Reflectins: the unusual proteins of squid reflective tissues. Science 303, 235–238 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

