Connecting the Dots: Therapy-Induced Senescence and a Tumor-Suppressive Immune Microenvironment
- PMID: 26719346
- PMCID: PMC4849355
- DOI: 10.1093/jnci/djv406
Connecting the Dots: Therapy-Induced Senescence and a Tumor-Suppressive Immune Microenvironment
Abstract
Background: Tumor cell senescence is a common outcome of anticancer therapy. Here we investigated how therapy-induced senescence (TIS) affects tumor-infiltrating leukocytes (TILs) and the efficacy of immunotherapy in melanoma.
Methods: Tumor senescence was induced by AURKA or CDK4/6 inhibitors (AURKAi, CDK4/6i). Transcriptomes of six mouse tumors with differential response to AURKAi were analyzed by RNA sequencing, and TILs were characterized by flow cytometry. Chemokine RNA and protein expression were determined by quantitative real-time polymerase chain reaction and enzyme-linked immunosorbent assay. Therapeutic response was queried in immunodeficient mice, in mice with CCL5-deficient tumors, and in mice cotreated with CD137 agonist to activate TILs. CCL5 expression in reference to TIS and markers of TILs was studied in human melanoma tumors using patient-derived xenografts (n = 3 patients, n = 3 mice each), in AURKAi clinical trial samples (n = 3 patients, before/after therapy), and in The Cancer Genome Atlas (n = 278). All statistical tests were two-sided.
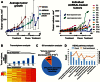
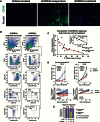
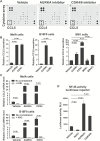
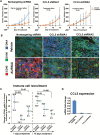
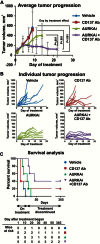
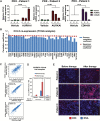
Results: AURKAi response was associated with induction of the immune transcriptome (P = 3.5 x 10-29) while resistance inversely correlated with TIL numbers (Spearman r = -0.87, P < .001). AURKAi and CDK4/6i promoted the recruitment of TILs by inducing CCL5 secretion in melanoma cells (P ≤ .005) in an NF-κB-dependent manner. Therapeutic response to AURKAi was impaired in immunodeficient compared with immunocompetent mice (0% vs 67% tumors regressed, P = .01) and in mice bearing CCL5-deficient vs control tumors (P = .61 vs P = .02); however, AURKAi response was greatly enhanced in mice also receiving T-cell-activating immunotherapy (P < .001). In human tumors, CCL5 expression was also induced by AURKAi (P ≤ .02) and CDK4/6i (P = .01) and was associated with increased immune marker expression (P = 1.40 x 10-93).
Conclusions: Senescent melanoma cells secret CCL5, which promotes recruitment of TILs. Combining TIS with immunotherapy that enhances tumor cell killing by TILs is a promising novel approach to improve melanoma outcomes.
© The Author 2015. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372(1):30–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

