Understanding TRPV1 activation by ligands: Insights from the binding modes of capsaicin and resiniferatoxin
- PMID: 26719417
- PMCID: PMC4720335
- DOI: 10.1073/pnas.1517288113
Understanding TRPV1 activation by ligands: Insights from the binding modes of capsaicin and resiniferatoxin
Abstract
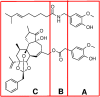
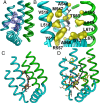
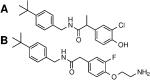
The transient receptor potential cation channel subfamily V member 1 (TRPV1) or vanilloid receptor 1 is a nonselective cation channel that is involved in the detection and transduction of nociceptive stimuli. Inflammation and nerve damage result in the up-regulation of TRPV1 transcription, and, therefore, modulators of TRPV1 channels are potentially useful in the treatment of inflammatory and neuropathic pain. Understanding the binding modes of known ligands would significantly contribute to the success of TRPV1 modulator drug design programs. The recent cryo-electron microscopy structure of TRPV1 only provides a coarse characterization of the location of capsaicin (CAPS) and resiniferatoxin (RTX). Herein, we use the information contained in the experimental electron density maps to accurately determine the binding mode of CAPS and RTX and experimentally validate the computational results by mutagenesis. On the basis of these results, we perform a detailed analysis of TRPV1-ligand interactions, characterizing the protein ligand contacts and the role of individual water molecules. Importantly, our results provide a rational explanation and suggestion of TRPV1 ligand modifications that should improve binding affinity.
Keywords: docking; heat-sensitive; ligand-gated; nociception; vanilloid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
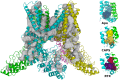


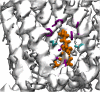







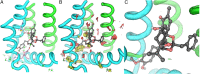
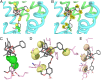
References
-
- Vriens J, Nilius B, Voets T. Peripheral thermosensation in mammals. Nat Rev Neurosci. 2014;15(9):573–589. - PubMed
-
- Clapham DE, Julius D, Montell C, Schultz G. International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev. 2005;57(4):427–450. - PubMed
-
- Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–647. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

