An Immune-Inflammation Gene Expression Signature in Prostate Tumors of Smokers
- PMID: 26719530
- PMCID: PMC4775384
- DOI: 10.1158/0008-5472.CAN-14-3630
An Immune-Inflammation Gene Expression Signature in Prostate Tumors of Smokers
Abstract
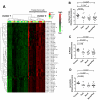
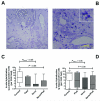
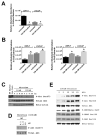
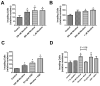
Smokers develop metastatic prostate cancer more frequently than nonsmokers, suggesting that a tobacco-derived factor is driving metastatic progression. To identify smoking-induced alterations in human prostate cancer, we analyzed gene and protein expression patterns in tumors collected from current, past, and never smokers. By this route, we elucidated a distinct pattern of molecular alterations characterized by an immune and inflammation signature in tumors from current smokers that were either attenuated or absent in past and never smokers. Specifically, this signature included elevated immunoglobulin expression by tumor-infiltrating B cells, NF-κB activation, and increased chemokine expression. In an alternate approach to characterize smoking-induced oncogenic alterations, we also explored the effects of nicotine in human prostate cancer cells and prostate cancer-prone TRAMP mice. These investigations showed that nicotine increased glutamine consumption and invasiveness of cancer cells in vitro and accelerated metastatic progression in tumor-bearing TRAMP mice. Overall, our findings suggest that nicotine is sufficient to induce a phenotype resembling the epidemiology of smoking-associated prostate cancer progression, illuminating a novel candidate driver underlying metastatic prostate cancer in current smokers.
©2015 American Association for Cancer Research.
Figures


References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J.Clin. 2011;61:69–90. - PubMed
-
- Hsing AW, Chokkalingam AP. Prostate cancer epidemiology. Front Biosci. 2006;11:1388–1413. - PubMed
-
- Giovannucci E, Rimm EB, Ascherio A, Colditz GA, Spiegelman D, Stampfer MJ, Willett WC. Smoking and risk of total and fatal prostate cancer in United States health professionals. Cancer Epidemiol.Biomarkers Prev. 1999;8:277–282. - PubMed
-
- Hickey K, Do KA, Green A. Smoking and prostate cancer. Epidemiol.Rev. 2001;23:115–125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

