MicroRNA-125a-5p regulates cancer cell proliferation and migration through NAIF1 in prostate carcinoma
- PMID: 26719710
- PMCID: PMC4689268
- DOI: 10.2147/OTT.S92314
MicroRNA-125a-5p regulates cancer cell proliferation and migration through NAIF1 in prostate carcinoma
Abstract
Background: We investigated the functional roles of microRNA-125a-5p in regulating human prostate carcinoma.
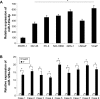
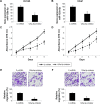
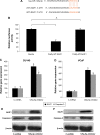
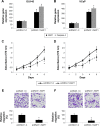
Methods: Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was conducted to evaluate the gene expression levels of miR-125a-5p in eight prostate cancer cell lines and nine biopsy specimens from patients with prostate cancer. miR-125a-5p was genetically knocked down in prostate cancer cell lines, DU145 and VCaP cells by lentiviral transduction. The effects of miR-125a-5p downregulation on prostate cancer cell proliferation and migration were evaluated by MTT assay and transwell assay, respectively. Direct regulation of miR-125a-5p on its downstream targets, NAIF1, and apoptotic gene caspase-3 were evaluated through dual-luciferase reporter assay, qRT-PCR, and Western blot, respectively. NAIF1 was then ectopically overexpressed in DU145 and VCaP cells to modulate prostate cancer cell proliferation and migration. Finally, the effects of miR-125a-5p downregulation or NAIF1 overexpression on the growth of in vivo prostate cancer xenograft were evaluated.
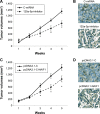
Results: miR-125a-5p was upregulated in prostate cancer cell lines and human prostate carcinomas. Lentivirus induced miR-125a-5p downregulation in DU145 and VCaP cells inhibited prostate cancer cell proliferation or migration. NAIF1 was the direct target of miR-125a-5p, as both gene and protein expression levels of NAIF1, as well as caspase-3 were upregulated by miR-125a-5p. Forced overexpression of NAIF1 had similar antitumor effects as miR-125a-5p downregulation on prostate cancer cell proliferation and migration. In vivo prostate xenograft assay confirmed the tumor-suppressive effect of miR-125a-5p downregulation or NAIF1 overexpression.
Conclusion: miR-125a-5p regulates prostate cancer cell proliferation and migration through NAIF1.
Keywords: NAIF1; caspase-3; miR-125a-5p; migration; proliferation.
Figures
Similar articles
-
MicroRNA-125a-5p modulates human cervical carcinoma proliferation and migration by targeting ABL2.Drug Des Devel Ther. 2015 Dec 24;10:71-9. doi: 10.2147/DDDT.S93104. eCollection 2016. Drug Des Devel Ther. 2015. PMID: 26766902 Free PMC article.
-
miRNA-125a-5p inhibits hepatocellular carcinoma cell proliferation and induces apoptosis by targeting TP53 regulated inhibitor of apoptosis 1 and Bcl-2-like-2 protein.Exp Ther Med. 2019 Aug;18(2):1196-1202. doi: 10.3892/etm.2019.7674. Epub 2019 Jun 14. Exp Ther Med. 2019. PMID: 31316614 Free PMC article.
-
MiR-125a-5p inhibits the proliferation and invasion of breast cancer cells and induces apoptosis by targeting GAB2.Math Biosci Eng. 2019 Jul 29;16(6):6923-6933. doi: 10.3934/mbe.2019347. Math Biosci Eng. 2019. PMID: 31698596
-
MicroRNA-125a-5p plays a role as a tumor suppressor in lung carcinoma cells by directly targeting STAT3.Tumour Biol. 2017 Jun;39(6):1010428317697579. doi: 10.1177/1010428317697579. Tumour Biol. 2017. PMID: 28631574
-
miR-125a-5p inhibits colorectal cancer cell epithelial-mesenchymal transition, invasion and migration by targeting TAZ.Onco Targets Ther. 2019 May 7;12:3481-3489. doi: 10.2147/OTT.S191247. eCollection 2019. Onco Targets Ther. 2019. PMID: 31190857 Free PMC article.
Cited by
-
Identification of reference microRNAs in skeletal muscle of a canine model of Duchenne muscular dystrophy.Wellcome Open Res. 2024 Nov 20;9:362. doi: 10.12688/wellcomeopenres.22481.2. eCollection 2024. Wellcome Open Res. 2024. PMID: 39649621 Free PMC article.
-
miR‑125a‑5p reverses epithelial‑mesenchymal transition and restores drug sensitivity by negatively regulating TAFAZZIN signaling in breast cancer.Mol Med Rep. 2021 Nov;24(5):812. doi: 10.3892/mmr.2021.12452. Epub 2021 Sep 22. Mol Med Rep. 2021. PMID: 34549308 Free PMC article.
-
Curcumol Inhibits the Development of Prostate Cancer by miR-125a/STAT3 Axis.Evid Based Complement Alternat Med. 2022 Jul 30;2022:9317402. doi: 10.1155/2022/9317402. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35942374 Free PMC article.
-
NAIF1 suppresses osteosarcoma progression and is regulated by miR-128.Cell Biochem Funct. 2018 Dec;36(8):443-449. doi: 10.1002/cbf.3365. Epub 2018 Nov 8. Cell Biochem Funct. 2018. PMID: 30407643 Free PMC article.
-
miR-125a-5p Functions as Tumor Suppressor microRNA And Is a Marker of Locoregional Recurrence And Poor prognosis in Head And Neck Cancer.Neoplasia. 2019 Sep;21(9):849-862. doi: 10.1016/j.neo.2019.06.004. Epub 2019 Jul 18. Neoplasia. 2019. PMID: 31325708 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

