Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue
- PMID: 26719857
- PMCID: PMC4687432
- DOI: 10.3892/ijmm.2015.2413
Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue
Abstract
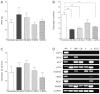
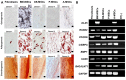
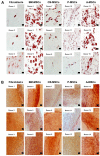
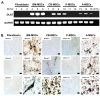
Mesenchymal stem cells (MSCs) are clinically useful due to their capacity for self-renewal, their immunomodulatory properties and tissue regenerative potential. These cells can be isolated from various tissues and exhibit different potential for clinical applications according to their origin, and thus comparative studies on MSCs from different tissues are essential. In this study, we investigated the immunophenotype, proliferative potential, multilineage differentiation and immunomodulatory capacity of MSCs derived from different tissue sources, namely bone marrow, adipose tissue, the placenta and umbilical cord blood. The gene expression profiles of stemness-related genes [octamer-binding transcription factor 4 (OCT4), sex determining region Y-box (SOX)2, MYC, Krüppel-like factor 4 (KLF4), NANOG, LIN28 and REX1] and lineage‑related and differentiation stage-related genes [B4GALNT1 (GM2/GS2 synthase), inhibin, beta A (INHBA), distal-less homeobox 5 (DLX5), runt-related transcription factor 2 (RUNX2), proliferator‑activated receptor gamma (PPARG), CCAAT/enhancer-binding protein alpha (C/EBPA), bone morphogenetic protein 7 (BMP7) and SOX9] were compared using RT-PCR. No significant differences in growth rate, colony-forming efficiency and immunophenotype were observed. Our results demonstrated that MSCs derived from bone marrow and adipose tissue shared not only in vitro tri-lineage differentiation potential, but also gene expression profiles. While there was considerable inter-donor variation in DLX5 expression between MSCs derived from different tissues, its expression appears to be associated with the osteogenic potential of MSCs. Bone marrow-derived MSCs (BM-MSCs) significantly inhibited allogeneic T cell proliferation possibly via the high levels of the immunosuppressive cytokines, IL10 and TGFB1. Although MSCs derived from different tissues and fibroblasts share many characteristics, some of the marker genes, such as B4GALNT1 and DLX5 may be useful for the characterization of MSCs derived from different tissue sources. Collectively, our results suggest that, based on their tri-lineage differentiation potential and immunomodulatory effects, BM-MSCs and adipose tissue-derived MSCs (A-MSCs) represent the optimal stem cell source for tissue engineering and regenerative medicine.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

