How Does the VSG Coat of Bloodstream Form African Trypanosomes Interact with External Proteins?
- PMID: 26719972
- PMCID: PMC4697842
- DOI: 10.1371/journal.ppat.1005259
How Does the VSG Coat of Bloodstream Form African Trypanosomes Interact with External Proteins?
Abstract
Variations on the statement "the variant surface glycoprotein (VSG) coat that covers the external face of the mammalian bloodstream form of Trypanosoma brucei acts a physical barrier" appear regularly in research articles and reviews. The concept of the impenetrable VSG coat is an attractive one, as it provides a clear model for understanding how a trypanosome population persists; each successive VSG protects the plasma membrane and is immunologically distinct from previous VSGs. What is the evidence that the VSG coat is an impenetrable barrier, and how do antibodies and other extracellular proteins interact with it? In this review, the nature of the extracellular surface of the bloodstream form trypanosome is described, and past experiments that investigated binding of antibodies and lectins to trypanosomes are analysed using knowledge of VSG sequence and structure that was unavailable when the experiments were performed. Epitopes for some VSG monoclonal antibodies are mapped as far as possible from previous experimental data, onto models of VSG structures. The binding of lectins to some, but not to other, VSGs is revisited with more recent knowledge of the location and nature of N-linked oligosaccharides. The conclusions are: (i) Much of the variation observed in earlier experiments can be explained by the identity of the individual VSGs. (ii) Much of an individual VSG is accessible to antibodies, and the barrier that prevents access to the cell surface is probably at the base of the VSG N-terminal domain, approximately 5 nm from the plasma membrane. This second conclusion highlights a gap in our understanding of how the VSG coat works, as several plasma membrane proteins with large extracellular domains are very unlikely to be hidden from host antibodies by VSG.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
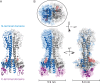


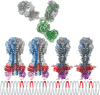
References
-
- Chattopadhyay A, Jones N, Nietlispach D, Nielsen P, Voorheis H, et al. (2005) Structure of the C-terminal Domain from Trypanosoma brucei Variant Surface Glycoprotein MITat1.2. J Biol Chem 280: 7227–7235. - PubMed
-
- Jones NG, Nietlispach D, Sharma R, Burke DF, Eyres I, et al. (2008) Structure of a glycosylphosphatidylinositol-anchored domain from a trypanosome variant surface glycoprotein. J Biol Chem 283: 3584–3593. - PubMed
-
- Mehlert A, Bond CS, Ferguson MA (2002) The glycoforms of a Trypanosoma brucei variant surface glycoprotein and molecular modeling of a glycosylated surface coat. Glycobiology 12: 607–612. - PubMed
-
- Carrington M, Miller N, Blum M, Roditi I, Wiley D, et al. (1991) Variant specific glycoprotein of Trypanosoma brucei consists of two domains each having an independently conserved pattern of cysteine residues. J Mol Biol 221: 823–835. - PubMed
-
- Blum ML, Down JA, Gurnett AM, Carrington M, Turner MJ, et al. (1993) A structural motif in the variant surface glycoproteins of Trypanosoma brucei. Nature 362: 603–609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

