Glycans from Fasciola hepatica Modulate the Host Immune Response and TLR-Induced Maturation of Dendritic Cells
- PMID: 26720149
- PMCID: PMC4697847
- DOI: 10.1371/journal.pntd.0004234
Glycans from Fasciola hepatica Modulate the Host Immune Response and TLR-Induced Maturation of Dendritic Cells
Abstract
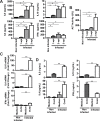
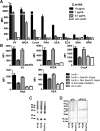
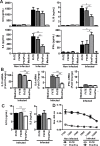
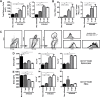
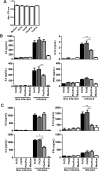
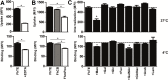
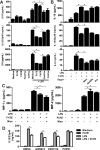
Helminths express various carbohydrate-containing glycoconjugates on their surface, and they release glycan-rich excretion/secretion products that can be very important in their life cycles, infection and pathology. Recent evidence suggests that parasite glycoconjugates could play a role in the evasion of the immune response, leading to a modified Th2-polarized immune response that favors parasite survival in the host. Nevertheless, there is limited information about the nature or function of glycans produced by the trematode Fasciola hepatica, the causative agent of fasciolosis. In this paper, we investigate whether glycosylated molecules from F. hepatica participate in the modulation of host immunity. We also focus on dendritic cells, since they are an important target of immune-modulation by helminths, affecting their activity or function. Our results indicate that glycans from F. hepatica promote the production of IL-4 and IL-10, suppressing IFNγ production. During infection, this parasite is able to induce a semi-mature phenotype of DCs expressing low levels of MHCII and secrete IL-10. Furthermore, we show that parasite glycoconjugates mediate the modulation of LPS-induced maturation of DCs since their oxidation restores the capacity of LPS-treated DCs to secrete high levels of the pro-inflammatory cytokines IL-6 and IL-12/23p40 and low levels of the anti-inflammatory cytokine IL-10. Inhibition assays using carbohydrates suggest that the immune-modulation is mediated, at least in part, by the recognition of a mannose specific-CLR that signals by recruiting the phosphatase Php2. The results presented here contribute to the understanding of the role of parasite glycosylated molecules in the modulation of the host immunity and might be useful in the design of vaccines against fasciolosis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Dowling D, Hamilton C, Donnelly S, La Course J, Brophy P, Dalton J, et al. Major secretory antigens of the helminth Fasciola hepatica activate a suppressive dendritic cell phenotype that attenuates Th17 cells but fails to activate Th2 immune responses. Infect Immun. 2010;78:793–801. 10.1128/IAI.00573-09 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

