Generating iPSC-Derived Choroidal Endothelial Cells to Study Age-Related Macular Degeneration
- PMID: 26720480
- PMCID: PMC5110238
- DOI: 10.1167/iovs.15-17073
Generating iPSC-Derived Choroidal Endothelial Cells to Study Age-Related Macular Degeneration
Abstract
Purpose: Age-related macular degeneration (AMD), the most common cause of incurable blindness in the western world, is characterized by the dysfunction and eventual death of choroidal endothelial (CECs), RPE, and photoreceptor cells. Stem cell-based treatment strategies designed to replace photoreceptor and RPE cells currently are a major scientific focus. However, the success of these approaches likely also will require replacement of the underlying, supportive choroidal vasculature. The purpose of this study was to generate stem cell-derived CECs to develop efficient differentiation and transplantation protocols.
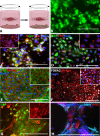
Methods: Dermal fibroblasts from the Tie2-GFP mouse were isolated and reprogrammed into two independent induced pluripotent stem cell (iPSC) lines via viral transduction of the transcription factors Oct4, Sox2, Klf4, and c-Myc. Tie2-GFP iPSCs were differentiated into CECs using a coculture method with either the RF6A CEC line or primary mouse CECs. Induced pluripotent stem cell-derived CECs were characterized via RT-PCR and immunocytochemistry for EC- and CEC-specific markers.
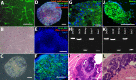
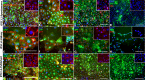
Results: Induced pluripotent stem cells generated from mice expressing green fluorescent protein (GFP) under control of the endothelial Tie2 promoter display classic pluripotency markers and stem cell morphology. Induced pluripotent stem cell-derived CECs express carbonic anhydrase IV, eNOS, FOXA2, PLVAP, CD31, CD34, ICAM-1, Tie2, TTR, VE-cadherin, and vWF.
Conclusions: Induced pluripotent stem cell-derived CECs will be a valuable tool for modeling of choriocapillaris-specific insults in AMD and for use in future choroidal endothelial cell replacement approaches.
Figures


References
-
- Buch H,, Vinding T,, La Cour M,, Nielsen NV. The prevalence and causes of bilateral and unilateral blindness in an elderly urban Danish population. The Copenhagen City Eye Study. Acta Ophthalmol Scand. 2001; 79: 441–449. - PubMed
-
- Smith W,, Assink J,, Klein R,, et al. Risk factors for age-related macular degeneration: pooled findings from three continents. Ophthalmology. 2001; 108: 697–704. - PubMed
-
- Friedman DS,, O'Colmain BJ,, Muñoz B,, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004; 122: 564–572. - PubMed
-
- VanNewkirk MR,, Nanjan MB,, Wang JJ,, Mitchell P,, Taylor HR,, McCarty CA. The prevalence of age-related maculopathy: the visual impairment project. Ophthalmology. 2000; 107: 1593–1600. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

