Stem cell-based gene therapy activated using magnetic hyperthermia to enhance the treatment of cancer
- PMID: 26720500
- PMCID: PMC4715938
- DOI: 10.1016/j.biomaterials.2015.11.023
Stem cell-based gene therapy activated using magnetic hyperthermia to enhance the treatment of cancer
Abstract
Stem cell-based gene therapies, wherein stem cells are genetically engineered to express therapeutic molecules, have shown tremendous potential for cancer applications owing to their innate ability to home to tumors. However, traditional stem cell-based gene therapies are hampered by our current inability to control when the therapeutic genes are actually turned on, thereby resulting in detrimental side effects. Here, we report the novel application of magnetic core-shell nanoparticles for the dual purpose of delivering and activating a heat-inducible gene vector that encodes TNF-related apoptosis-inducing ligand (TRAIL) in adipose-derived mesenchymal stem cells (AD-MSCs). By combining the tumor tropism of the AD-MSCs with the spatiotemporal MCNP-based delivery and activation of TRAIL expression, this platform provides an attractive means with which to enhance our control over the activation of stem cell-based gene therapies. In particular, we found that these engineered AD-MSCs retained their innate ability to proliferate, differentiate, and, most importantly, home to tumors, making them ideal cellular carriers. Moreover, exposure of the engineered AD-MSCS to mild magnetic hyperthermia resulted in the selective expression of TRAIL from the engineered AD-MSCs and, as a result, induced significant ovarian cancer cell death in vitro and in vivo.
Keywords: Cancer therapy; Gene therapy; Hyperthermia; Magnetic core–shell nanoparticles; Stem cell therapy.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures

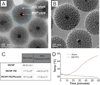



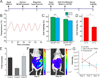
Comment in
-
Technical Advances in Oncology Outside of Radiation Medicine.Int J Radiat Oncol Biol Phys. 2016 Aug 1;95(5):1323-1326. doi: 10.1016/j.ijrobp.2016.02.066. Int J Radiat Oncol Biol Phys. 2016. PMID: 27479719 No abstract available.
References
-
- Bevis KS, Buchsbaum DJ, Straughn JM. Overcoming TRAIL resistance in ovarian carcinoma. Gynecol Oncol. 2010;119:157–163. - PubMed
-
- Aletti GD, Gallenberg MM, Cliby WA, Jatoi A, Hartmann LC. Current management strategies for ovarian cancer. Mayo Clin Proc. 2007;82:751–770. - PubMed
-
- McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. New Engl J Med. 1996;334:1–6. - PubMed
-
- Peer D, Karp JM, Hong S, FaroKHzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

