The BD FACSPresto Point of Care CD4 Test Accurately Enumerates CD4+ T Cell Counts
- PMID: 26720601
- PMCID: PMC4697849
- DOI: 10.1371/journal.pone.0145586
The BD FACSPresto Point of Care CD4 Test Accurately Enumerates CD4+ T Cell Counts
Erratum in
-
Correction: The BD FACSPresto Point of Care CD4 Test accurately enumerates CD4+ T cell counts.PLoS One. 2016 Dec 9;11(12):e0167667. doi: 10.1371/journal.pone.0167667. eCollection 2016. PLoS One. 2016. PMID: 27936057 Free PMC article.
Abstract
Objective: Currently 50% of ART eligible patients are not yet receiving life-saving antiretroviral therapy (ART). Financial constraints do not allow most developing countries to adopt a universal test and offer ART strategy. Decentralizing CD4+ T cell testing may, therefore, provide greater access to testing, ART, and better patient management. We evaluated the technical performance of a new point-of-care CD4+ T cell technology, the BD FACSPresto, in a field methods comparison study.
Methods: 264 HIV-positive patients were consecutively enrolled and included in the study. The BD FACSPresto POC CD4+ T cell technology was placed in two rural health care facilities and operated by health care facility staff. We compared paired finger-prick and venous samples using the BD FACSPresto and several existing reference technologies, respectively.
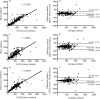
Results: The BD FACSPresto had a mean bias of 67.29 cells/ul and an r(2) of 0.9203 compared to the BD FACSCalibur. At ART eligibility thresholds of 350 and 500 cells/ul, the sensitivity to define treatment eligibility were 81.5% and 77.2% and the specificities were 98.9% and 100%, respectively. Similar results were observed when the BD FACSPresto was compared to the BD FACSCount and Alere Pima. The coefficient of variation (CV) was less than 7% for both the BD FACSCalibur and BD FACSPresto. CD4+ T cell testing by nurses using the BD FACSPresto at rural health care facilities showed high technical similarity to test results generated by laboratory technicians using the BD FACSPresto in a high functioning laboratory.
Conclusions: The BD FACSPresto performed favorably in the laboratory setting compared to the conventional reference standard technologies; however, the lower sensitivities indicated that up to 20% of patients tested in the field in need of treatment would be missed. The BD FACSPresto is a technology that can allow for greater decentralization and wider access to CD4+ T cell testing and ART.
Conflict of interest statement
Figures
References
-
- UNAIDS (2014) The Gap Report.
-
- UNITAID (2014) HIV/AIDS Diagnostic Technology Landscape, 4th edition.
-
- Jani IV, Sitoe NE, Alfai ER, Chongo PL, Quevedo JI, Rocha BM, et al. (2011) Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: an observational cohort study. Lancet 378: 1572–1579. 10.1016/S0140-6736(11)61052-0 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials