Occludin deficiency promotes ethanol-induced disruption of colonic epithelial junctions, gut barrier dysfunction and liver damage in mice
- PMID: 26721332
- PMCID: PMC4776745
- DOI: 10.1016/j.bbagen.2015.12.013
Occludin deficiency promotes ethanol-induced disruption of colonic epithelial junctions, gut barrier dysfunction and liver damage in mice
Abstract
Background: Disruption of epithelial tight junctions (TJ), gut barrier dysfunction and endotoxemia play crucial role in the pathogenesis of alcoholic tissue injury. Occludin, a transmembrane protein of TJ, is depleted in colon by alcohol. However, it is unknown whether occludin depletion influences alcoholic gut and liver injury.
Methods: Wild type (WT) and occludin deficient (Ocln(-/-)) mice were fed 1-6% ethanol in Lieber-DeCarli diet. Gut permeability was measured by vascular-to-luminal flux of FITC-inulin. Junctional integrity was analyzed by confocal microscopy. Liver injury was assessed by plasma transaminase, histopathology and triglyceride analyses. The effect of occludin depletion on acetaldehyde-induced TJ disruption was confirmed in Caco-2 cell monolayers.
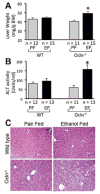
Results: Ethanol feeding significantly reduced body weight gain in Ocln(-/-) mice. Ethanol increased inulin permeability in colon of both WT and Ocln(-/-) mice, but the effect was 4-fold higher in Ocln(-/-) mice. The gross morphology of colonic mucosa was unaltered, but ethanol disrupted the actin cytoskeleton, induced redistribution of occludin, ZO-1, E-cadherin and β-catenin from the junctions and elevated TLR4, which was more severe in Ocln(-/-) mice. Occludin knockdown significantly enhanced acetaldehyde-induced TJ disruption and barrier dysfunction in Caco-2 cell monolayers. Ethanol significantly increased liver weight and plasma transaminase activity in Ocln(-/-) mice, but not in WT mice. Histological analysis indicated more severe lesions and fat deposition in the liver of ethanol-fed Ocln(-/-) mice. Ethanol-induced elevation of liver triglyceride was also higher in Ocln(-/-) mice.
Conclusion: This study indicates that occludin deficiency increases susceptibility to ethanol-induced colonic mucosal barrier dysfunction and liver damage in mice.
Keywords: Actin; Adherens junction; Alcohol; Claudin; Fatty liver; Tight junction.
Published by Elsevier B.V.
Figures







References
-
- Rao RK, Seth A, Sheth P. Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G881–884. - PubMed
-
- Atkinson KJ, Rao RK. Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1280–1288. - PubMed
-
- Ma TY, Nguyen D, Bui V, Nguyen H, Hoa N. Ethanol modulation of intestinal epithelial tight junction barrier. Am J Physiol. 1999;276:G965–974. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

