Evaluating and Reporting the Immunogenicity Impacts for Biological Products--a Clinical Pharmacology Perspective
- PMID: 26721560
- PMCID: PMC4779104
- DOI: 10.1208/s12248-015-9857-y
Evaluating and Reporting the Immunogenicity Impacts for Biological Products--a Clinical Pharmacology Perspective
Abstract
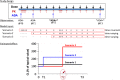
Immunogenicity assessment is important for biological products due to potential impacts of immunogenicity on safety and efficacy. We reviewed the prescribing information and the FDA's clinical pharmacology review of 121 approved biological products for evaluating and reporting of immunogenicity data. Of the 121 products, 89% (n = 108) reported the incidence of immunogenicity and 49% (n = 59) reported immunogenicity impact on efficacy. However, only 26% (n = 31) reported whether the immunogenicity affected pharmacokinetics. A subset of 16 products reported effects of anti-drug antibodies (ADA) on both systemic clearance and efficacy; 8 of 16 products had increased systemic clearance coinciding with reduced efficacy, and 6 of 16 products had no changes in either clearance or efficacy. Factors contributing to infrequent reporting of the ADA effect on exposure and methods for determining the effect of ADA on exposure are summarized. Measuring ADA and drug concentrations concurrently over time enables the evaluation of ADA impact on pharmacokinetics. Within-subject comparison of concentration data (before vs. after ADA formation) is a useful alternative to between-subject (ADA+ vs. ADA-) comparison when sample size is limited or when the majority of subjects developed ADA. The biological complexity of immune responses presents challenges to quantifying the ADA impact on pharmacokinetics using model-based methods. Our findings support that pharmacokinetic exposure is more sensitive than efficacy endpoints for evaluating ADA effects. A decrease in drug concentration due to formation of ADA during treatment can serve as an early indicator for potential reduced efficacy occurring at a later time.
Keywords: clinical pharmacology assessment; immunogenicity data for approved biological products; impact on clinical pharmacokinetics and efficacy; incidence of anti-drug antibodies and neutralizing antibodies.
Figures



References
-
- Shankar G, Arkin S, Cocea L, Devanarayan V, Kirshner S, Kromminga A, et al. Assessment and reporting of the clinical immunogenicity of therapeutic proteins and peptides-harmonized terminology and tactical recommendations. AAPS J. 2014;16(4):658–673. doi: 10.1208/s12248-014-9599-2. - DOI - PMC - PubMed
-
- FDA Guidance for Industry: Immunogenicity Assessment for Therapeutic Protein Products (2014).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

