Down-regulation of the mitochondrial matrix peptidase ClpP in muscle cells causes mitochondrial dysfunction and decreases cell proliferation
- PMID: 26721594
- PMCID: PMC5584630
- DOI: 10.1016/j.freeradbiomed.2015.12.021
Down-regulation of the mitochondrial matrix peptidase ClpP in muscle cells causes mitochondrial dysfunction and decreases cell proliferation
Abstract
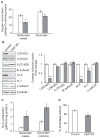
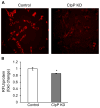
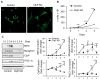
The caseinolytic peptidase P (ClpP) is the endopeptidase component of the mitochondrial matrix ATP-dependent ClpXP protease. ClpP degrades unfolded proteins to maintain mitochondrial protein homeostasis and is involved in the initiation of the mitochondrial unfolded protein response (UPR(mt)). Outside of an integral role in the UPR(mt), the cellular function of ClpP is not well characterized in mammalian cells. To investigate the role of ClpP in mitochondrial function, we generated C2C12 muscle cells that are deficient in ClpP using siRNA or stable knockdown using lentiviral transduction. Reduction of ClpP levels by ~70% in C2C12 muscle cells resulted in a number of mitochondrial alterations including reduced mitochondrial respiration and reduced oxygen consumption rate in response to electron transport chain (ETC) complex I and II substrates. The reduction in ClpP altered mitochondrial morphology, changed the expression level of mitochondrial fission protein Drp1 and blunted UPR(mt) induction. In addition, ClpP deficient cells showed increased generation of reactive oxygen species (ROS) and decreased membrane potential. At the cellular level, reduction of ClpP impaired myoblast differentiation, cell proliferation and elevated phosphorylation of eukaryotic initiation factor 2 alpha (eIF2α) suggesting an inhibition of translation. Our study is the first to define the effects of ClpP deficiency on mitochondrial function in muscle cells in vitro. In addition, we have uncovered novel effects of ClpP on mitochondrial morphology, cell proliferation and protein translation pathways in muscle cells.
Keywords: ClpP; ClpX; Mitochondrial fission/fusion; Mitochondrial unfolded protein response; Reactive oxygen species; Respiration.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures



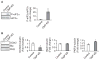
References
-
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. - PubMed
-
- Luce K, Weil AC, Osiewacz HD. Mitochondrial protein quality control systems in aging and disease. Adv Exp Med Biol. 2010;694:108–125. - PubMed
-
- Bota DA, Davies KJ. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat Cell Biol. 2002;4:674–680. - PubMed
-
- Truscott KN, Bezawork-Geleta A, Dougan DA. Unfolded protein responses in bacteria and mitochondria: A central role for the ClpXP machine. IUBMB Life. 2011;63:955–963. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

