Challenges in the development of therapeutics for narcolepsy
- PMID: 26721620
- PMCID: PMC5114175
- DOI: 10.1016/j.pneurobio.2015.12.002
Challenges in the development of therapeutics for narcolepsy
Abstract
Narcolepsy is a neurological disorder that afflicts 1 in 2000 individuals and is characterized by excessive daytime sleepiness and cataplexy-a sudden loss of muscle tone triggered by positive emotions. Features of narcolepsy include dysregulation of arousal state boundaries as well as autonomic and metabolic disturbances. Disruption of neurotransmission through the hypocretin/orexin (Hcrt) system, usually by degeneration of the HCRT-producing neurons in the posterior hypothalamus, results in narcolepsy. The cause of Hcrt neurodegeneration is unknown but thought to be related to autoimmune processes. Current treatments for narcolepsy are symptomatic, including wake-promoting therapeutics that increase presynaptic dopamine release and anticataplectic agents that activate monoaminergic neurotransmission. Sodium oxybate is the only medication approved by the US Food and Drug Administration that alleviates both sleep/wake disturbances and cataplexy. Development of therapeutics for narcolepsy has been challenged by historical misunderstanding of the disease, its many disparate symptoms and, until recently, its unknown etiology. Animal models have been essential to elucidating the neuropathology underlying narcolepsy. These models have also aided understanding the neurobiology of the Hcrt system, mechanisms of cataplexy, and the pharmacology of narcolepsy medications. Transgenic rodent models will be critical in the development of novel therapeutics for the treatment of narcolepsy, particularly efforts directed to overcome challenges in the development of hypocretin replacement therapy.
Keywords: Animal models; Cataplexy; Hypocretin; Narcolepsy; Neurodegeneration; Orexin.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures

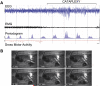

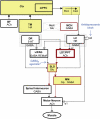

References
-
- Absalom N, Eghorn LF, Villumsen IS, Karim N, Bay T, Olsen JV, Knudsen GM, Brauner-Osborne H, Frolund B, Clausen RP, Chebib M, Wellendorph P. alpha4betadelta GABAA receptors are high-affinity targets for gamma-hydroxybutyric acid (GHB). Proc. Natl. Acad. Sci. U.S.A. 2012;109:13404–13409. - PMC - PubMed
-
- Ahmed SS, Schur PH, MacDonald NE, Steinman L. Narcolepsy, 2009 A(H1N1) pandemic influenza, and pandemic influenza vaccinations: what is known and unknown about the neurological disorder, the role for autoimmunity, and vaccine adjuvants. J. Autoimmun. 2014;50:1–11. - PubMed
-
- Ahmed SS, Volkmuth W, Duca J, Corti L, Pallaoro M, Pezzicoli A, Karle A, Rigat F, Rappuoli R, Narasimhan V, Julkunen I, Vuorela A, Vaarala O, Nohynek H, Pasini FL, Montomoli E, Trombetta C, Adams CM, Rothbard J, Steinman L. Antibodies to influenza nucleoprotein cross-react with human hypocretin receptor 2. Sci. Transl. Med. 2015;7:294ra105. - PubMed
-
- Akimoto H, Honda Y, Takahashi Y. Pharmacotherapy in narcolepsy. Dis. Nerv. Syst. 1960;21:704–706. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

