Toll-like Receptors in the Vascular System: Sensing the Dangers Within
- PMID: 26721702
- PMCID: PMC4709508
- DOI: 10.1124/pr.114.010090
Toll-like Receptors in the Vascular System: Sensing the Dangers Within
Abstract
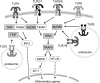
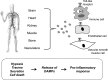
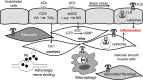
Toll-like receptors (TLRs) are components of the innate immune system that respond to exogenous infectious ligands (pathogen-associated molecular patterns, PAMPs) and endogenous molecules that are released during host tissue injury/death (damage-associated molecular patterns, DAMPs). Interaction of TLRs with their ligands leads to activation of downstream signaling pathways that induce an immune response by producing inflammatory cytokines, type I interferons (IFN), and other inflammatory mediators. TLR activation affects vascular function and remodeling, and these molecular events prime antigen-specific adaptive immune responses. Despite the presence of TLRs in vascular cells, the exact mechanisms whereby TLR signaling affects the function of vascular tissues are largely unknown. Cardiovascular diseases are considered chronic inflammatory conditions, and accumulating data show that TLRs and the innate immune system play a determinant role in the initiation and development of cardiovascular diseases. This evidence unfolds a possibility that targeting TLRs and the innate immune system may be a novel therapeutic goal for these conditions. TLR inhibitors and agonists are already in clinical trials for inflammatory conditions such as asthma, cancer, and autoimmune diseases, but their study in the context of cardiovascular diseases is in its infancy. In this article, we review the current knowledge of TLR signaling in the cardiovascular system with an emphasis on atherosclerosis, hypertension, and cerebrovascular injury. Furthermore, we address the therapeutic potential of TLR as pharmacological targets in cardiovascular disease and consider intriguing research questions for future study.
Copyright © 2015 by The American Society for Pharmacology and Experimental Therapeutics.
Figures


References
-
- Adachi N, Hirota M, Hamaguchi M, Okamoto K, Watanabe K, Endo F. (2004) Serum cytochrome c level as a prognostic indicator in patients with systemic inflammatory response syndrome. Clin Chim Acta 342:127–136. - PubMed
-
- Akira S, Uematsu S, Takeuchi O. (2006) Pathogen recognition and innate immunity. Cell 124:783–801. - PubMed
-
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. (2001) Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732–738. - PubMed
-
- An SJ, Liu P, Shao TM, Wang ZJ, Lu HG, Jiao Z, Li X, Fu JQ. (2015) Characterization and functions of vascular adventitial fibroblast subpopulations. Cell Physiol Biochem 35:1137–1150. - PubMed
-
- Anderson KV, Jürgens G, Nüsslein-Volhard C. (1985) Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell 42:779–789. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

