Deletion of the membrane complement inhibitor CD59a drives age and gender-dependent alterations to bone phenotype in mice
- PMID: 26721735
- PMCID: PMC4764651
- DOI: 10.1016/j.bone.2015.12.014
Deletion of the membrane complement inhibitor CD59a drives age and gender-dependent alterations to bone phenotype in mice
Abstract
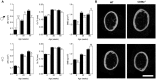
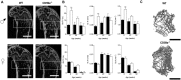
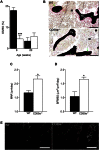
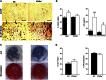
Degenerative joint diseases such as osteoarthritis are characterised by aberrant region-specific bone formation and abnormal bone mineral content. A recent study suggested a role for the complement membrane attack complex in experimental models of osteoarthritis. Since CD59a is the principal regulator of the membrane attack complex in mice, we evaluated the impact of CD59a gene deletion upon maintenance of bone architecture. In vivo bone morphology analysis revealed that male CD59a-deficient mice have increased femur length and cortical bone volume, albeit with reduced bone mineral density. However, this phenomenon was not observed in female mice. Histomorphometric analysis of the trabecular bone showed increased rates of bone homeostasis, with both increased bone resorption and mineral apposition rate in CD59a-deficient male mice. When bone cells were studied in isolation, in vitro osteoclastogenesis was significantly increased in male CD59a-deficient mice, although osteoblast formation was not altered. Our data reveal, for the first time, that CD59a is a regulator of bone growth and homeostasis. CD59a ablation in male mice results in longer and wider bones, but with less density, which is likely a major contributing factor for their susceptibility to osteoarthritis. These findings increase our understanding of the role of complement regulation in degenerative arthritis.
Keywords: Ageing; Bone; CD59a; Micro-CT; Osteoclast.
Copyright © 2016 Amgen Inc. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Brunetti G., Di Benedetto A., Mori G. Bone Remodeling. In: Albanse C.V., editor. Faletti C, editors. Springer; Imaging of Prosthetic Joints: 2014. pp. 27–37.
-
- Parra-Torres A.Y., Valdés-Flores M., Orozco L., Velázquez-Cruz R. Molecular aspects of bone remodeling. In: Flores M.V., editor. Topics in Osteoporosis. first ed. InTech; 2013.
-
- Verborgt O., Gibson G.J., Schaffler M.B. Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J. Bone Miner. Res. 2000;15:60–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

