Medium- to Long-Term Impact of Rotavirus Vaccination on Hospital Care in Belgium: A 7-Year Follow-Up of the Rotavirus Belgium Impact Study (RotaBIS)
- PMID: 26721823
- PMCID: PMC4811837
- DOI: 10.1007/s40121-015-0099-1
Medium- to Long-Term Impact of Rotavirus Vaccination on Hospital Care in Belgium: A 7-Year Follow-Up of the Rotavirus Belgium Impact Study (RotaBIS)
Abstract
Introduction: Rotavirus (RV) vaccination was introduced in Belgium in 2006. With the high uptake it had (>85%), a sharp decline in hospitalizations was observed during the first years after vaccine introduction. The objective of this study was to investigate whether this decline was maintained and to simulate projections.
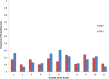
Methods: The Rotavirus Belgium Impact Study allowed an analysis of the RV vaccine impact amongst children in 11 hospitals in Belgium over a 9-year period (2005-2013) with 2 years pre- and 7 years post-vaccine introduction. Results were compared by year and by subsequent birth cohort aging up to 5 years. The two different analysis methods helped dismantling the different (direct and indirect) effects of vaccine protection to simulate future hospitalization trends.
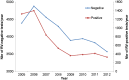
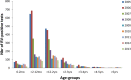
Results: During the whole observation period, 40,552 RV detection tests were performed of which 5832 were positive (14.4%). After RV vaccine introduction, a significant reduction in number of tests performed (-38%) was combined with a dramatic drop in numbers of positive tests (-76.6%). The decreases were spectacular during the first two years of vaccine introduction; after that period, the decrease flattened. Cross-sectional comparison with cohort data showed that the initial drop was heavily influenced by the herd effect of the vaccine. Cohort analysis demonstrated a low rate of residual disease over time, suggesting another infection source other than the child population.
Conclusion: The residual disease will be maintained in the community when a same vaccination strategy is continued over time, starting vaccination of children only at 6 weeks' time.
Funding: GlaxoSmithKline Biologicals SA.
Trial registration: ClinicalTrials.gov identifier, NCT01563146.
Keywords: Children; Gastroenteritis; Hospitalization; Rotavirus; Vaccination.
Figures

References
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

