CXCL1/MGSA Is a Novel Glycosaminoglycan (GAG)-binding Chemokine: STRUCTURAL EVIDENCE FOR TWO DISTINCT NON-OVERLAPPING BINDING DOMAINS
- PMID: 26721883
- PMCID: PMC4759198
- DOI: 10.1074/jbc.M115.697888
CXCL1/MGSA Is a Novel Glycosaminoglycan (GAG)-binding Chemokine: STRUCTURAL EVIDENCE FOR TWO DISTINCT NON-OVERLAPPING BINDING DOMAINS
Abstract
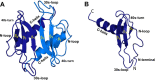
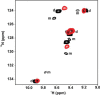
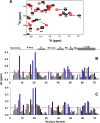
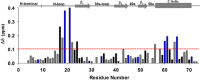
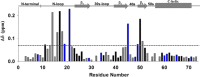
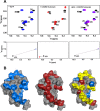
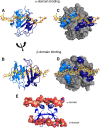
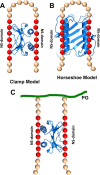
In humans, the chemokine CXCL1/MGSA (hCXCL1) plays fundamental and diverse roles in pathophysiology, from microbial killing to cancer progression, by orchestrating the directed migration of immune and non-immune cells. Cellular trafficking is highly regulated and requires concentration gradients that are achieved by interactions with sulfated glycosaminoglycans (GAGs). However, very little is known regarding the structural basis underlying hCXCL1-GAG interactions. We addressed this by characterizing the binding of GAG heparin oligosaccharides to hCXCL1 using NMR spectroscopy. Binding experiments under conditions at which hCXCL1 exists as monomers and dimers indicate that the dimer is the high-affinity GAG ligand. NMR experiments and modeling studies indicate that lysine and arginine residues mediate binding and that they are located in two non-overlapping domains. One domain, consisting of N-loop and C-helical residues (defined as α-domain) has also been identified previously as the GAG-binding domain for the related chemokine CXCL8/IL-8. The second domain, consisting of residues from the N terminus, 40s turn, and third β-strand (defined as β-domain) is novel. Eliminating β-domain binding by mutagenesis does not perturb α-domain binding, indicating two independent GAG-binding sites. It is known that N-loop and N-terminal residues mediate receptor activation, and we show that these residues are also involved in extensive GAG interactions. We also show that the GAG-bound hCXCL1 completely occlude receptor binding. We conclude that hCXCL1-GAG interactions provide stringent control over regulating chemokine levels and receptor accessibility and activation, and that chemotactic gradients mediate cellular trafficking to the target site.
Keywords: CXC chemokines; CXCL1/MGSA; G protein-coupled receptor (GPCR); NMR; cell migration; chemokine; glycobiology; glycosaminoglycan; heparin; immunology.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Bonecchi R., Galliera E., Borroni E. M., Corsi M. M., Locati M., and Mantovani A. (2009) Chemokines and chemokine receptors: an overview. Front. Biosci. 14, 540–551 - PubMed
-
- Griffith J. W., Sokol C. L., and Luster A. D. (2014) Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu. Rev. Immunol. 32, 659–702 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

