OS9 Protein Interacts with Na-K-2Cl Co-transporter (NKCC2) and Targets Its Immature Form for the Endoplasmic Reticulum-associated Degradation Pathway
- PMID: 26721884
- PMCID: PMC4813476
- DOI: 10.1074/jbc.M115.702514
OS9 Protein Interacts with Na-K-2Cl Co-transporter (NKCC2) and Targets Its Immature Form for the Endoplasmic Reticulum-associated Degradation Pathway
Abstract
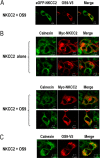
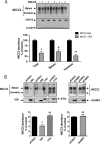
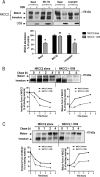
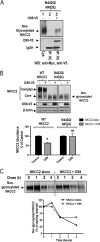
Mutations in the renal specific Na-K-2Cl co-transporter (NKCC2) lead to type I Bartter syndrome, a life-threatening kidney disease featuring arterial hypotension along with electrolyte abnormalities. We have previously shown that NKCC2 and its disease-causing mutants are subject to regulation by endoplasmic reticulum-associated degradation (ERAD). The aim of the present study was to identify the protein partners specifically involved in ERAD of NKCC2. To this end, we screened a kidney cDNA library through a yeast two-hybrid assay using NKCC2 C terminus as bait. We identified OS9 (amplified in osteosarcomas) as a novel and specific binding partner of NKCC2. Co-immunoprecipitation assays in renal cells revealed that OS9 association involves mainly the immature form of NKCC2. Accordingly, immunocytochemistry analysis showed that NKCC2 and OS9 co-localize at the endoplasmic reticulum. In cells overexpressing OS9, total cellular NKCC2 protein levels were markedly decreased, an effect blocked by the proteasome inhibitor MG132. Pulse-chase and cycloheximide-chase assays demonstrated that the marked reduction in the co-transporter protein levels was essentially due to increased protein degradation of the immature form of NKCC2. Conversely, knockdown of OS9 by small interfering RNA increased NKCC2 expression by increasing the co-transporter stability. Inactivation of the mannose 6-phosphate receptor homology domain of OS9 had no effect on its action on NKCC2. In contrast, mutations of NKCC2 N-glycosylation sites abolished the effects of OS9, indicating that OS9-induced protein degradation is N-glycan-dependent. In summary, our results demonstrate the presence of an OS9-mediated ERAD pathway in renal cells that degrades immature NKCC2 proteins. The identification and selective modulation of ERAD components specific to NKCC2 and its disease-causing mutants might provide novel therapeutic strategies for the treatment of type I Bartter syndrome.
Keywords: Na-K-Cl co-transporter (NKCC); endoplasmic reticulum-associated protein degradation (ERAD); hypertension; intracellular trafficking; kidney; membrane trafficking; sodium transport.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





Similar articles
-
AUP1 Regulates the Endoplasmic Reticulum-Associated Degradation and Polyubiquitination of NKCC2.Cells. 2024 Feb 24;13(5):389. doi: 10.3390/cells13050389. Cells. 2024. PMID: 38474353 Free PMC article.
-
New insights into the role of endoplasmic reticulum-associated degradation in Bartter Syndrome Type 1.Hum Mutat. 2021 Aug;42(8):947-968. doi: 10.1002/humu.24217. Epub 2021 May 31. Hum Mutat. 2021. PMID: 33973684
-
Differential Effects of STCH and Stress-Inducible Hsp70 on the Stability and Maturation of NKCC2.Int J Mol Sci. 2021 Feb 23;22(4):2207. doi: 10.3390/ijms22042207. Int J Mol Sci. 2021. PMID: 33672238 Free PMC article.
-
Protein Quality Control of NKCC2 in Bartter Syndrome and Blood Pressure Regulation.Cells. 2024 May 10;13(10):818. doi: 10.3390/cells13100818. Cells. 2024. PMID: 38786040 Free PMC article. Review.
-
Na+-K+-2Cl- cotransporter type 2 trafficking and activity: the role of interacting proteins.Biol Cell. 2012 Apr;104(4):201-12. doi: 10.1111/boc.201100049. Epub 2012 Jan 26. Biol Cell. 2012. PMID: 22211456 Review.
Cited by
-
Redundant and Antagonistic Roles of XTP3B and OS9 in Decoding Glycan and Non-glycan Degrons in ER-Associated Degradation.Mol Cell. 2018 May 3;70(3):516-530.e6. doi: 10.1016/j.molcel.2018.03.026. Epub 2018 Apr 26. Mol Cell. 2018. PMID: 29706535 Free PMC article.
-
The expression system influences stability, maturation efficiency, and oligomeric properties of the potassium-chloride co-transporter KCC2.Neurochem Int. 2024 Mar;174:105695. doi: 10.1016/j.neuint.2024.105695. Epub 2024 Feb 17. Neurochem Int. 2024. PMID: 38373478 Free PMC article.
-
Golgi Alpha1,2-Mannosidase IA Promotes Efficient Endoplasmic Reticulum-Associated Degradation of NKCC2.Cells. 2021 Dec 29;11(1):101. doi: 10.3390/cells11010101. Cells. 2021. PMID: 35011665 Free PMC article.
-
IL-17A deficiency mitigates bleomycin-induced complement activation during lung fibrosis.FASEB J. 2017 Dec;31(12):5543-5556. doi: 10.1096/fj.201700289R. Epub 2017 Aug 17. FASEB J. 2017. PMID: 28821630 Free PMC article.
-
AUP1 Regulates the Endoplasmic Reticulum-Associated Degradation and Polyubiquitination of NKCC2.Cells. 2024 Feb 24;13(5):389. doi: 10.3390/cells13050389. Cells. 2024. PMID: 38474353 Free PMC article.
References
-
- Greger R., and Velázquez H. (1987) The cortical thick ascending limb and early distal convoluted tubule in the urinary concentrating mechanism. Kidney Int. 31, 590–596 - PubMed
-
- Gamba G. (2005) Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol. Rev. 85, 423–493 - PubMed
-
- Gamba G. (1999) Molecular biology of distal nephron sodium transport mechanisms. Kidney Int. 56, 1606–1622 - PubMed
-
- Wu J., Liu X., Lai G., Yang X., Wang L., and Zhao Y. (2013) Synergistical effect of 20-HETE and high salt on NKCC2 protein and blood pressure via ubiquitin-proteasome pathway. Hum. Genet. 132, 179–187 - PubMed
-
- Kim D., Sands J. M., and Klein J. D. (2004) Role of vasopressin in diabetes mellitus-induced changes in medullary transport proteins involved in urine concentration in Brattleboro rats. Am. J. Physiol. Renal Physiol. 286, F760–F766 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

