Rac1 Signaling Is Required for Anterior Second Heart Field Cellular Organization and Cardiac Outflow Tract Development
- PMID: 26722124
- PMCID: PMC4859369
- DOI: 10.1161/JAHA.115.002508
Rac1 Signaling Is Required for Anterior Second Heart Field Cellular Organization and Cardiac Outflow Tract Development
Abstract
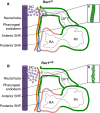
Background: The small GTPase Rac1 regulates diverse cellular functions, including both apicobasal and planar cell polarity pathways; however, its role in cardiac outflow tract (OFT) development remains unknown. In the present study, we aimed to examine the role of Rac1 in the anterior second heart field (SHF) splanchnic mesoderm and subsequent OFT development during heart morphogenesis.
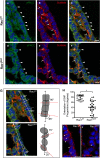
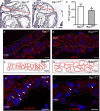
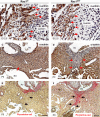
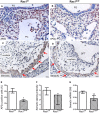
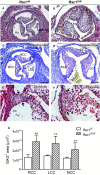
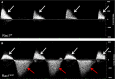
Methods and results: Using the Cre/loxP system, mice with an anterior SHF-specific deletion of Rac1 (Rac1(SHF)) were generated. Embryos were collected at various developmental time points for immunostaining and histological analysis. Intrauterine echocardiography was also performed to assess aortic valve blood flow in embryos at embryonic day 18.5. The Rac1(SHF) splanchnic mesoderm exhibited disruptions in SHF progenitor cellular organization and proliferation. Consequently, this led to a spectrum of OFT defects along with aortic valve defects in Rac1(SHF) embryos. Mechanistically, it was found that the ability of the Rac1(SHF) OFT myocardial cells to migrate into the proximal OFT cushion was severely reduced. In addition, expression of the neural crest chemoattractant semaphorin 3c was decreased. Lineage tracing showed that anterior SHF contribution to the OFT myocardium and aortic valves was deficient in Rac1(SHF) hearts. Furthermore, functional analysis with intrauterine echocardiography at embryonic day 18.5 showed aortic valve regurgitation in Rac1(SHF) hearts, which was not seen in control hearts.
Conclusions: Disruptions of Rac1 signaling in the anterior SHF results in aberrant progenitor cellular organization and defects in OFT development. Our data show Rac1 signaling to be a critical regulator of cardiac OFT formation during embryonic heart development.
Keywords: Rac1; cellular organization; congenital heart defect; outflow tract development.
© 2015 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures




References
-
- Bruneau BG. The developmental genetics of congenital heart disease. Nature. 2008;451:943–948. - PubMed
-
- Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI. Consensus‐based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2002;123:110–118. - PubMed
-
- Phillips HM, Rhee HJ, Murdoch JN, Hildreth V, Peat JD, Anderson RH, Copp AJ, Chaudhry B, Henderson DJ. Disruption of planar cell polarity signaling results in congenital heart defects and cardiomyopathy attributable to early cardiomyocyte disorganization. Circ Res. 2007;101:137–145. - PubMed
-
- Henderson DJ, Phillips HM, Chaudhry B. Vang‐like 2 and noncanonical Wnt signaling in outflow tract development. Trends Cardiovasc Med. 2006;16:38–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

